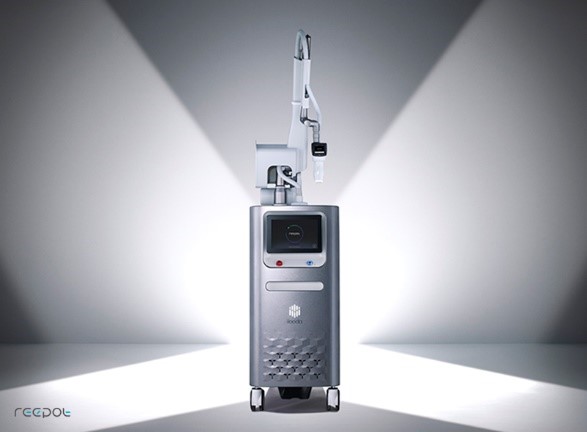
[Asia Economy Reporter Chunhee Lee] Iruda, a specialized company in beauty medical devices, announced on the 26th that its next-generation laser device 'Reepot (reepot Nd:YAG laser system)' has received 510(k) approval from the U.S. Food and Drug Administration (FDA).
This achievement follows the product approval obtained from the Korean Ministry of Food and Drug Safety in April. The company plans to actively pursue additional approvals overseas, including Europe, starting with this approval.
Reepot is a device applying 'VSLS' technology to a 532 nm wavelength Q-Switched Nd:YAG laser source. Following 'SecretRF,' it is Iruda's signature equipment that will drive the company's next-generation growth. In Korea, a launch event was held for medical professionals on the 17th, and full-scale sales are currently underway.
Kim Yonghan, CEO of Iruda, stated, "The FDA approval for Reepot was achieved faster than expected, so we will completely revise our initial plan to target the U.S. market and respond quickly to market demands." He added, "Once the European CE approval is completed, we expect Reepot to bring innovation to the global laser medical device market."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.