Existing treatments 'Allopurinol', 'Febuxostat', etc.
Serious side effects in cardiovascular system and skin
LG Chem's 'Tigulixostat' and JW Chungwoo's 'URC102'
No safety issues up to Phase 2 clinical trials
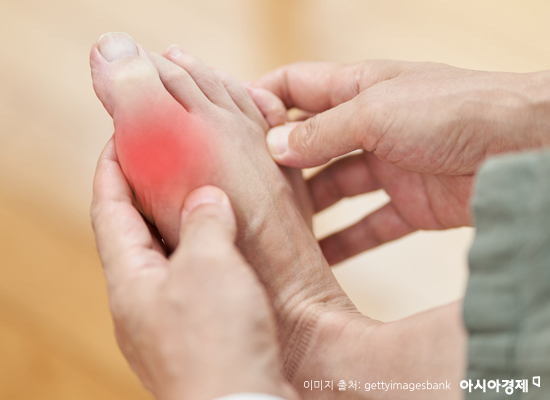
[Asia Economy Reporter Lee Chun-hee] #Lee Min-gyu (36) developed gout in his foot at a young age. Although he was prescribed medication, the doctor warned that there could be side effects such as heart attacks. While taking the prescribed medicine due to severe pain, he cannot help but continue worrying about heart disease.
The number of gout patients, who say "even a breeze hurts," is rapidly increasing. Especially due to the westernization of dietary habits, the incidence rate among younger people is also rising significantly. According to the Health Insurance Review and Assessment Service on the 16th, the number of gout patients in Korea was 395,154 in 2017 and nearly reached 500,000 last year at 492,373. In particular, patients aged 30 and under jumped by a staggering 43.2%, from 90,918 to 131,500 during the same period.
This rapid increase in gout patients is not only seen in Korea but worldwide. Market research firm Grand View Research forecasts that the global gout treatment market will grow at an average annual rate of 16.1%, reaching $8.3 billion (approximately 11.6 trillion KRW) by 2025.
However, most currently used gout treatments come with serious side effects, creating a significant unmet need for safe alternative therapies. The main active ingredients used for gout treatment are 'allopurinol' and 'febuxostat.' Allopurinol was developed first in the 1960s and has been mainly used since then. Febuxostat, approved by the U.S. Food and Drug Administration (FDA) in 2009, showed superior efficacy compared to allopurinol and emerged as a first-line treatment. However, due to concerns about side effects such as cardiovascular disease, the FDA has excluded febuxostat from first-line treatment options.
Allopurinol also has considerable side effects, including causing severe skin diseases. Moreover, the risk factor gene ‘HLA-B*5801’ that can trigger these side effects is present in only 0.7% of Caucasians, but 7.4% of Southeast Asians, 3.8% of African Americans, and about 12% of Koreans. Therefore, without prior genetic testing, prescribing allopurinol in Korea is difficult.
Can a domestic treatment without side effects emerge?
On the other hand, new gout drugs being developed domestically are gaining attention as no serious adverse events related to safety have been reported so far. LG Chem’s ‘Tigulixostat’ and JW Pharmaceutical’s ‘URC102’ are representative examples. Both drugs have completed Phase 2 clinical trials and, although still in Phase 2, no significant safety issues have arisen.
Tigulixostat is more advanced, having already applied for Phase 3 clinical trials. After submitting an Investigational New Drug (IND) application to the FDA last month, it also applied for Phase 3 trials in China on the 12th. In terms of efficacy, in the previous Phase 2 trial, the 200 mg dosage group achieved a 62.2% rate of reaching the primary endpoint?serum uric acid levels below 5 mg/dL after 3 months of administration?demonstrating superior results compared to febuxostat’s 23.1%. If Phase 3 confirms this efficacy without serious adverse events, Tigulixostat has a high potential to become a global first-line treatment.

JW Pharmaceutical’s URC102 targets the market with a different mechanism. URC102 is a uric acid excretion promoter. While allopurinol and febuxostat target ‘overproduction-type’ gout caused by excessive uric acid production, patients with ‘underexcretion-type’ gout, caused by insufficient uric acid excretion, require uric acid excretion promoters. However, currently developed drugs like ‘Lesinurad’ and ‘Benzbromarone’ are used only in limited countries due to kidney or liver toxicity issues.
If URC102’s development proceeds smoothly, it could capture this essentially untapped market. In the Phase 2b trial completed last year, the 9 mg dosage group achieved an 88.9% rate of reaching the primary endpoint?serum uric acid levels below 6 mg/dL after 4 weeks?surpassing febuxostat’s 84.2%. Safety and tolerability were confirmed to be at the same level as the placebo group.
JW Pharmaceutical plans to apply for Phase 3 clinical trials of URC102 in Korea within this year. Chinese pharmaceutical company Simcere, which licensed the technology in 2019 for up to $70 million (approximately 97.8 billion KRW), has completed Phase 1 trials and is preparing for subsequent clinical trials.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















