[Asia Economy Reporter Kim Young-won] 'Skycovione,' the first domestically produced vaccine, has shown effectiveness against the BA.1 and BA.5 variants after booster vaccination.
On the 2nd, the National Institute of Infectious Diseases at the Korea National Institute of Health announced that cross-neutralization ability against the domestically prevalent Omicron variants BA.1 and BA.5 was confirmed after booster vaccination with SK Bioscience's COVID-19 vaccine Skycovione.
The Korea National Institute of Health conducted analysis of clinical phase 3 trial specimens required for the approval of Skycovione, including cross-neutralization ability analysis against the Delta variant and Omicron variant (BA.1).
The analysis subjects were classified into five groups according to the primary vaccination (1st and 2nd doses) vaccine. The five groups' primary vaccines were four single-dose groups of AstraZeneca, Pfizer, Moderna, and Janssen, and one heterologous vaccination group of AstraZeneca-Pfizer.
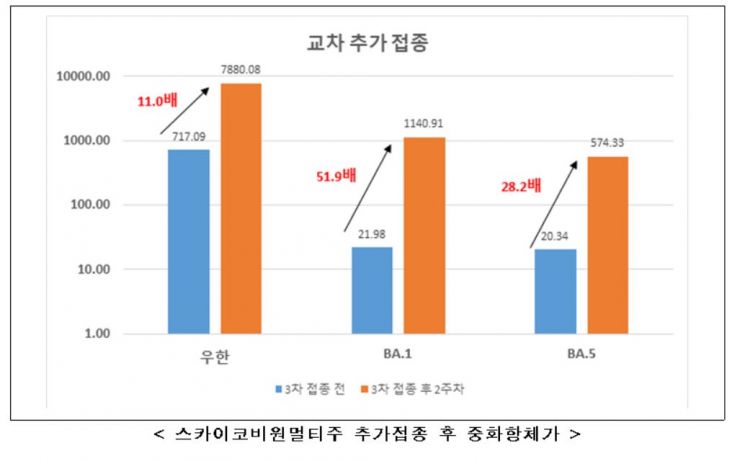
Two weeks after booster vaccination with Skycovione, analysis of the five groups showed that all five exhibited improved neutralization ability compared to before vaccination. Compared to before the booster, neutralization ability increased on average about 51.9-fold for BA.1 and about 28.2-fold for BA.5. Neutralizing antibody titers against the original Wuhan strain increased 11.0-fold.
In particular, the group primed with messenger RNA (mRNA) vaccines showed higher neutralization ability than other groups against the original Wuhan strain as well as BA.1 and BA.5.
Kwon Jun-wook, director of the Korea National Institute of Health, stated, "Skycovione Multi was developed as the first domestic COVID-19 vaccine through clinical trial specimen analysis support by the Korea National Institute of Health and the International Vaccine Institute. In the situation where variant viruses such as BA.5 continue to emerge, this analysis of variant strains in the booster group confirmed the potential for additional use of domestically developed vaccines."
Jang Hee-chang, director of the National Institute of Infectious Diseases, said, "We will continue to support clinical trial specimen analysis and variant virus evaluation for various platforms of domestically developed vaccines, including mRNA vaccines and viral vector vaccines."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.