[Asia Economy Reporter Chunhee Lee] GNT Pharma announced on the 29th that the Independent Data Monitoring Committee (IDMC) has recommended proceeding with the ongoing Phase 3 clinical trial of the stroke treatment drug 'Nellonemdaz' in China without any changes to the plan.
The IDMC is a group of experts who independently monitor patient safety and drug efficacy during ongoing clinical trials. They typically advise the trial sponsors on whether to continue the trial, modify the trial design, or terminate the trial. Usually, the IDMC conducts interim reviews during Phase 3 trials, as these trials require significant time and cost, and if the drug is ineffective, patients might miss the opportunity for treatment.
The IDMC conducted an in-depth evaluation of the interim results regarding the efficacy and safety of Nellonemdaz in 227 stroke patients participating in the Phase 3 trial and recommended proceeding with the remaining Phase 3 trial without modifying the trial design. The Phase 3 trial in China is being conducted by Apeloa Pharmaceutical, GNT Pharma's partner company in China. The trial aims to enroll a total of 948 patients, with 323 patients (34%) currently enrolled. The trial targets severe stroke patients who received the thrombolytic agent plasminogen activator (tPA) within 8 hours of onset to verify the final efficacy of Nellonemdaz.
GNT Pharma's independently conducted Phase 3 trial of Nellonemdaz in Korea is also progressing rapidly. It targets 496 severe acute ischemic stroke patients who undergo thrombectomy within 12 hours of onset to verify Nellonemdaz's neuroprotective effects and disability improvement. So far, 211 patients have been enrolled, showing a 43% progress rate. Patient enrollment is planned to be completed by mid-next year.
GNT Pharma plans to successfully complete the ongoing Phase 3 trials domestically and internationally and launch the world's first treatment for stroke patients who have undergone vascular recanalization therapy by 2025.
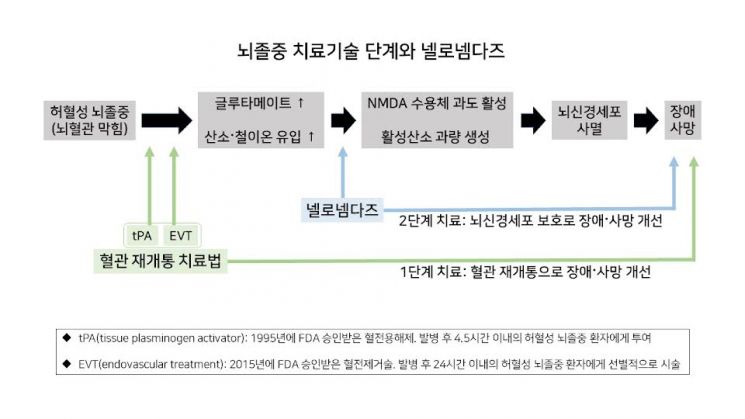
Nellonemdaz is an N-methyl-D-aspartate (NMDA) glutamate receptor antagonist that blocks calcium neurotoxicity while simultaneously removing reactive oxygen species generated in brain neurons, making it a multi-target neuroprotective drug. Existing NMDA glutamate receptor antagonists have side effects such as neurotoxicity and inducing schizophrenia-like symptoms, but Nellonemdaz has demonstrated safety in clinical trials involving 165 healthy adults and 447 acute stroke patients.
In a domestic Phase 2 clinical trial involving 209 stroke patients who underwent thrombectomy within 8 hours of onset, the Nellonemdaz treatment group showed significant improvement in the three major stroke efficacy evaluation indicators (disability assessment, activities of daily living assessment, and neurological assessment) compared to the placebo group. In-depth analysis of severe patients also clearly showed Nellonemdaz's disability improvement effects.
Denis Choi, Professor of Neurology at Stony Brook University School of Medicine in the United States, who identified the key mechanism of neuronal death after stroke, said, "The world is focusing on developing effective neuroprotective drugs that reduce disability and death in ischemic stroke patients, and the Phase 3 clinical trials of Nellonemdaz underway in Korea and China are at the center of this effort. I hope Nellonemdaz will pioneer a new foundation for treating stroke, which leads to permanent disability and death."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.