Estimated Domestic Supply for 28 Million People
Flu Season Earlier Than Usual
Pharmaceutical Industry Accelerates Production and Distribution
MFDS Supports National Release Approval
[Asia Economy Reporter Lee Gwan-joo] As the daily COVID-19 cases exceed 180,000 and the resurgence reaches its peak, concerns are growing about a ‘twindemic’?the simultaneous outbreak of COVID-19 and influenza (flu) this fall. The pharmaceutical industry anticipates an earlier flu season than usual and has begun rapid vaccine supply efforts.
According to health authorities and the pharmaceutical industry on the 19th, approximately 28 million doses of flu vaccine will be supplied domestically this year. Of these, 12 to 13 million doses will be used for national immunization programs targeting the elderly, pregnant women, and children, while the remainder will be distributed to private hospitals and clinics.
Typically, health authorities and the medical community expect the flu season to peak in November and promote vaccinations accordingly, with most vaccinations concentrated in October. However, this year, it is widely expected that the flu season will arrive earlier than usual. In 2020 and last year, strong quarantine measures implemented due to the COVID-19 pandemic also helped suppress the flu season. But with the lifting of social distancing measures, the flu season may be brought forward. Especially if COVID-19 and the flu, which have similar symptoms, spread simultaneously, the burden on health authorities and medical professionals will inevitably increase.
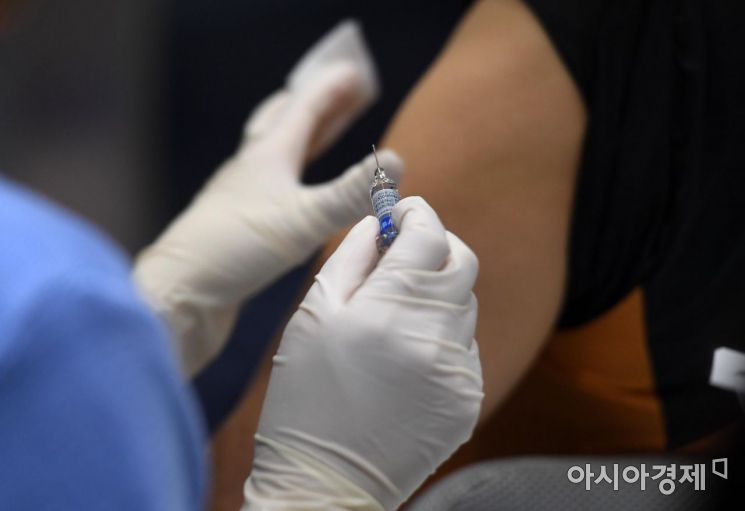
Considering this situation, the pharmaceutical industry has accelerated the production and distribution of flu vaccines. Seven companies in total manufacture flu vaccines distributed domestically: five Korean pharmaceutical companies and two foreign companies. Korean pharmaceutical companies obtained national release approval from the Ministry of Food and Drug Safety (MFDS) between the 16th and 18th. National release approval is a system where the MFDS verifies the safety and efficacy of each manufacturing batch of vaccines and approves their sale, especially for vaccines requiring special health precautions. Obtaining this approval means the vaccines will soon be distributed to the market.
By company, GC Green Cross’s ‘GC Flu Multi Injection’ received national release approval for two manufacturing batches on the 18th, and Boryung Biopharma’s ‘Boryung Flu V Tetra Vaccine Injection’ obtained approval for three batches on the 16th and 18th. Other flu vaccines such as Boryung (formerly Boryung Pharmaceutical) ‘BR Flu Tech I Tetra Vaccine Injection,’ Korea Vaccine’s ‘Kovax Flu Quadrivalent PF Injection,’ and Ilyang Pharmaceutical’s ‘Teratect Prefilled Syringe Injection’ have also received national release approval, so full-scale distribution is expected to begin next week. Hospitals and clinics receiving these vaccines plan to prepare and start full-scale flu vaccinations from September.
Two imported vaccines have also begun supply. Sanofi Korea obtained national release approval for Sanofi Pasteur’s ‘Vaxigrip Tetra Injection’ on the 17th and started distribution nationwide. Kwangdong Pharmaceutical, responsible for distributing GlaxoSmithKline (GSK)’s flu vaccine ‘Fluarix Tetra Prefilled Syringe’ domestically, has also entered full-scale sales. Fluarix Tetra is known as the world’s first quadrivalent flu vaccine approved by the U.S. Food and Drug Administration (FDA) and the first approved by the Korean MFDS. A Kwangdong Pharmaceutical official stated, “We will ensure stable and smooth supply so that nationwide vaccinations can begin in September,” adding, “We also plan to expand supply volumes to all hospitals and clinics, including pediatrics, obstetrics and gynecology, internal medicine, family medicine, and orthopedics.”
The MFDS is supporting manufacturers and importers in various ways to ensure rapid flu vaccine supply. For example, if vaccines with the same manufacturing number as those already approved are imported on different days, testing is waived. Also, when finished pharmaceuticals are continuously manufactured using the same final bulk solution, content testing is exempted from the second batch onward. These measures help shorten release times and enable rapid vaccine supply.
Meanwhile, the MFDS has set vaccination rate targets for this year’s national flu immunization program at 85% for seniors aged 65 and over, 55% for pregnant women, and 80% for children. Children requiring two doses can start vaccination from September 21, and those requiring one dose from October 5. Pregnant women’s vaccinations begin on October 5, and seniors aged 65 and over will be vaccinated sequentially from October 12, depending on age. Eligible individuals can receive free flu vaccinations at designated medical institutions or public health centers nearby.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)














