[Asia Economy Yeongnam Reporting Headquarters Reporter Hwang Du-yeol] Precise synthesis methods that assemble substances at the molecular level often require expensive metal catalysts and multi-step chemical reactions.
To reduce synthesis costs, developing synthesis methods that do not use precious metal catalysts and reduce reaction steps is important. A joint research team from the Department of Chemistry at UNIST has developed a chemical synthesis method that does not use precious metal catalysts.
The research team led by Professors Hong Seong-yu and Jan-Uwe Rohde at UNIST recently developed a method to synthesize 'nitrogen-containing nano graphene fragments' through radical chain reactions.
The material is attracting attention as a precise compound and as a next-generation polymer and energy material.
The developed synthesis method allows radicals to replace the role of catalysts, eliminating the need for expensive rare metal catalysts.
Radicals are substances that have unpaired electrons, and due to their tendency to pair these unpaired electrons, they exhibit high reactivity and can substitute for catalysts.
Previously, a pretreatment process attaching chemical functional groups to carbon rings was necessary to enhance catalyst efficiency, but such pretreatment is no longer required.
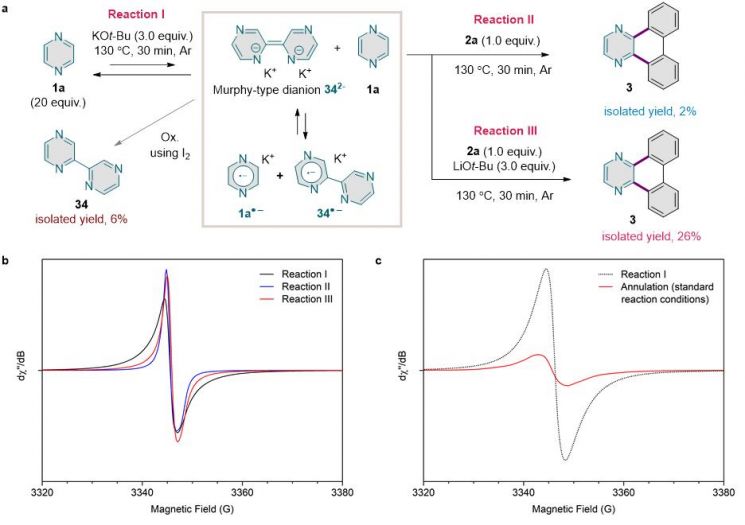
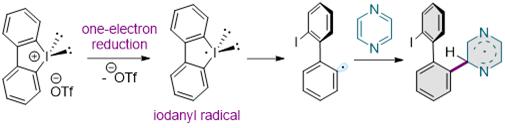
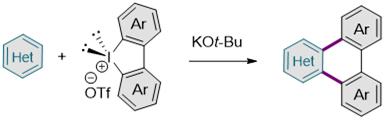
The research team chemically assembled azine series molecules containing nitrogen in the carbon ring and diaryliodonium salts to synthesize nitrogen-containing nano graphene fragments using the synthesis method.
They also elucidated the precise synthesis process through spectroscopic analysis capable of directly observing the presence of radical unpaired electrons.
Professor Hong Seong-yu stated, “This is a case where azine precursors, known for their low reactivity, were effectively utilized through radical reactions without using palladium catalysts,” adding, “This approach could also be applied to the development of new chemical syntheses.”
The synthesis part of the research involved Dr. Lee Jae-bin, Jung Seo-young, and PhD candidate Jeon Ji-hwan from the Department of Chemistry.
The research team explained, “Compared to methods that break down raw materials into smaller parts, this is a revolutionary synthesis method that can produce nitrogen-containing nano graphene fragments in a single-step reaction without complicated, time-consuming, and costly multi-step reactions.”
Spectroscopic analysis was led by PhD candidate Kim Geon-ha. Kim explained, “Using Electron Paramagnetic Resonance (EPR) Spectroscopy, which can directly observe the presence of unpaired electrons, we were able to reveal the principles of the synthesis method based on chain radical reactions.”
Professor Jan-Uwe Rohde said, “This is an excellent example of two research groups from different specialties closely collaborating to develop a new chemical reaction.”
The research was supported by the National Research Foundation of Korea and the Electronics and Telecommunications Research Institute (ETRI).
The research results were published in the multidisciplinary specialized journal Nature Communications.
 (From the bottom left, counterclockwise) Professor Jan Rode, Dr. Jaebin Lee, first author, Professor Seongyu Hong, researcher Geonha Kim, researcher Jihwan Jeon, researcher Seoyoung Jung.
(From the bottom left, counterclockwise) Professor Jan Rode, Dr. Jaebin Lee, first author, Professor Seongyu Hong, researcher Geonha Kim, researcher Jihwan Jeon, researcher Seoyoung Jung.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.