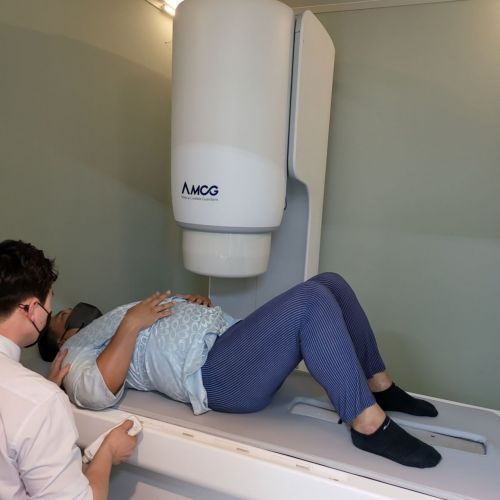
 AMCG research institute officials are demonstrating the inspection process using equipment applied with the Simjado system.
AMCG research institute officials are demonstrating the inspection process using equipment applied with the Simjado system.
AMCG, a specialist company in cardiac diagnostic devices, announced on the 27th that it has obtained medical device approval from the Ministry of Food and Drug Safety for its Magneto Cardio Graph (MCG) system.
The Magneto Cardio Graph (MCG) system measures biomagnetic signals generated by patients without using radiation or contrast agents. It can detect various heart abnormalities and arrhythmias at an early stage, and predict conditions such as fetal heart disease, arrhythmia, ischemia, and sudden cardiac death, which were previously difficult to diagnose or detect. Additionally, it offers the advantage of innovative technology enabling three-dimensional real-time examination, overcoming the limitations of existing diagnostic methods.
Originating in the United States in 1963, this technology was developed domestically over 20 years by Dr. Yongho Lee’s team at the Korea Research Institute of Standards and Science, which completed technology transfer to AMCG in March last year for commercialization. Overseas, products from companies such as Hitachi in Japan, Genetisis (U.S.A.), and CMI exist, but most have fewer than 64 channels, so in terms of resolution and sensitivity, the domestic technology is evaluated to be at least five years ahead.
Heart disease is the second leading cause of death after cancer and is a serious issue, but despite extensive global research, diagnostic technology for the heart has not significantly advanced. The electrocardiogram (ECG), developed in 1903, is still used for basic diagnosis even after more than 100 years, and to compensate for its inaccurate diagnosis, echocardiography, computed tomography (CT), and magnetic resonance imaging (MRI) are used as supplementary methods.
AMCG recently signed a contract for a large-scale factory site to begin full-scale production of equipment applying the MCG system and will soon start multi-center clinical trials to establish accurate diagnostic standards. It is also preparing to apply for U.S. Food and Drug Administration (FDA) approval for entry into the U.S. market in the second half of this year.
Seoyong Sung, Vice President of AMCG, stated, "Utilizing AMCG’s MCG equipment for early screening could dramatically reduce the number of heart disease patients, currently over 1.5 million annually in Korea, and more than 30,000 deaths caused by heart disease." He added, "After closely reviewing related technologies and market conditions for the past two years, the domestic cardiac diagnostic device market is estimated to be worth over 5 trillion KRW, and globally about 100 trillion KRW. We aim to commercialize the supplemented and developed core MCG technology within two years."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















