"Effectiveness Confirmed in NRAS-Mutant Melanoma Patient Relapsed After Immune Checkpoint Inhibitor Therapy"
[Asia Economy Reporter Lee Gwan-joo] Genentech, a subsidiary of global pharmaceutical company Roche, has disclosed the clinical progress of Hanmi Pharmaceutical's innovative anticancer drug 'belvarafenib,' currently under follow-up development, and expressed its intention for commercialization.
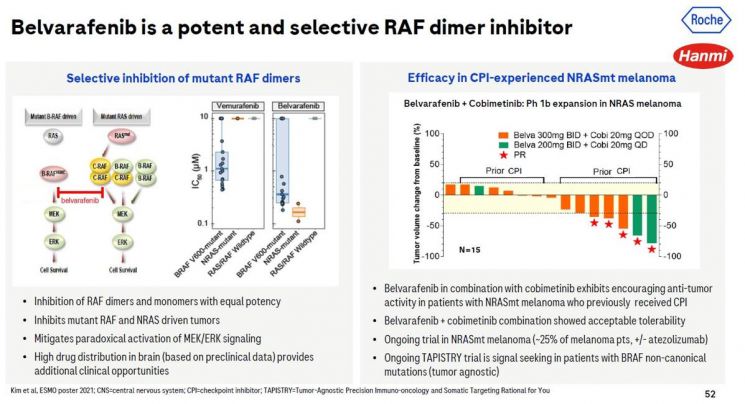
Hanmi Pharmaceutical announced on the 22nd that data on the efficacy and safety of belvarafenib combined with cobimetinib (a MEK inhibitor) in NRAS-mutant melanoma patients, confirmed through a clinical trial (1b), were presented at Roche's corporate presentation held at the American Society of Clinical Oncology (ASCO).
This clinical trial involved 118 patients with various types of solid tumors harboring RAF or RAS mutations, and the presentation was conducted by Andrew Chan, Executive Director in charge of RED (Research & Early Development) at Genentech.
According to the presentation, partial response (PR) was observed in 26.3% of NRAS-mutant melanoma patients treated with belvarafenib and cobimetinib, 42.1% of patients achieved stable disease (SD), and the median progression-free survival (PFS) was 7.3 months. No new adverse reactions beyond the safety profiles of the individual drugs were observed.
All patients who showed partial response were NRAS-mutant melanoma patients with a history of prior immune checkpoint inhibitor treatment. Hanmi Pharmaceutical explained that these results could offer new hope for patients who relapsed after immune checkpoint inhibitor therapy.
Andrew Chan, Executive Director at Genentech, stated, “Belvarafenib developed by Hanmi Pharmaceutical is a potent selective RAF mutation inhibitor,” adding, “We confirmed safety and antitumor efficacy when combined with cobimetinib in NRAS-mutant melanoma patients previously treated with immune checkpoint inhibitors.”
A Hanmi Pharmaceutical official said, “Innovative anticancer drugs developed and licensed out by Hanmi Pharmaceutical are progressing smoothly based on close consultations with partner companies,” and added, “We will further focus our R&D capabilities to accelerate the commercialization of various innovative new drugs, including belvarafenib.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.