KIST-POSTECH Joint Research Team
[Asia Economy Reporter Kim Bong-su] For South Korea to achieve carbon emission net zero by 2050, importing large quantities of hydrogen from overseas is essential. Domestic researchers have developed key technology necessary for transporting large volumes of hydrogen.
On the 12th, the Korea Institute of Science and Technology (KIST) announced that Dr. Son Hyun-tae of the Hydrogen and Fuel Cell Research Center, in collaboration with Professor Yoon Chang-won of the Department of Chemical Engineering at Pohang University of Science and Technology (POSTECH), developed a porous silica-based nanocatalyst required for the hydrogen extraction process of Liquid Organic Hydrogen Carriers (LOHC), which can store large amounts of hydrogen and transport it at room temperature and atmospheric pressure.
According to the first Hydrogen Economy Implementation Basic Plan announced by the government in November last year, South Korea aims to increase domestic hydrogen supply to 3.9 million tons by 2030, but plans to import more than half of that amount, 1.96 million tons, produced overseas. However, since hydrogen is compressed and transported domestically by ship, the amount of hydrogen that can be imported at one time is limited. This is why recent technology has attracted attention.
The catalyst developed by the research team drastically reduces the amount of byproducts generated during the hydrogen extraction process while accelerating the extraction speed, making it expected to become a core technology enabling large-scale hydrogen transport demonstrations in the future.
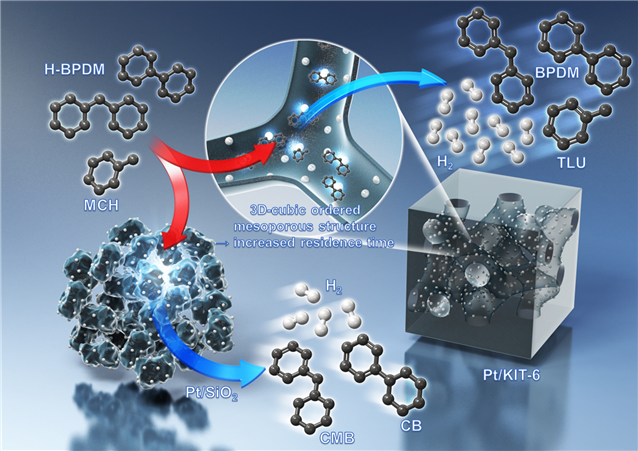
LOHC is a technology that uses organic compounds as a medium for hydrogen storage, transport, and release, enabling the transport of large volumes of hydrogen. It has properties similar to diesel and gasoline, allowing existing petrochemical facility infrastructure to be utilized without initial investment costs. Unlike liquid-based transport using ammonia, it is possible to repeat the hydrogen storage and extraction cycle, which can reduce costs. However, a small amount of partial dehydrogenation byproducts generated during the hydrogen extraction process accumulate during repeated storage-extraction cycles, reducing hydrogen storage capacity and overall process efficiency. Additionally, the catalyst’s stability decreases at the high temperatures of the hydrogen extraction process, lowering hydrogen production speed.
The catalyst developed by the research team consists of 1-2 nanometer (1nm: one billionth of a meter) platinum (Pt) metal evenly dispersed on three-dimensional ordered mesoporous silica (KIT-6). It recorded about 2.2 times higher dehydrogenation performance compared to the commercial catalyst Pt/Al2O3, and the biphenyl-based LOHC dehydrogenation byproducts were generated at about 1/20 the level of the commercial catalyst in the liquid product distribution. Furthermore, because the nano platinum metal particles exist inside each pore of the three-dimensional porous silica support, the catalyst remains stable even at high reaction temperatures and maintains performance after long-term use.
Dr. Son said, “This study improved hydrogen selectivity and production speed by controlling the pore size of the catalyst and the residence time of the biphenyl-based LOHC reactant,” adding, “The goal is to apply this catalyst to various LOHC extraction processes beyond biphenyl-based ones through further research.”
The results of this study were published in the latest issue of the international academic journal in the energy and environment field, ‘Applied Catalysis B-Environmental’ (IF: 19.503, JCR top 0.926%).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.