Interview with Taegyu Kim, CEO of ByzenCell
"Existing CAR-T therapies require patient-specific autologous production. VigenCell is developing universal cell therapies based on gamma delta T cells that can be mass-produced."
On the 20th, Kim Taegyu, CEO of VigenCell, expressed confidence that by developing universal cell and gene therapies, the company will establish itself as a next-generation leading biopharmaceutical. Cell and gene therapies have the potential to treat rare genetic diseases and intractable diseases that were previously difficult to cure, surpassing antibody therapies. Novartis's chimeric antigen receptor (CAR)-T cell therapy 'Kymriah' for leukemia was included in the health insurance reimbursement list starting last month. According to global market research firm Frost & Sullivan, the cell therapy market is expected to grow at an average annual rate of 36.2% to reach $4.6842 billion by 2026, while the gene therapy market is projected to grow at an average annual rate of 27.6% to reach $5.4292 billion.
Challenging Blood and Solid Cancers with T Cells
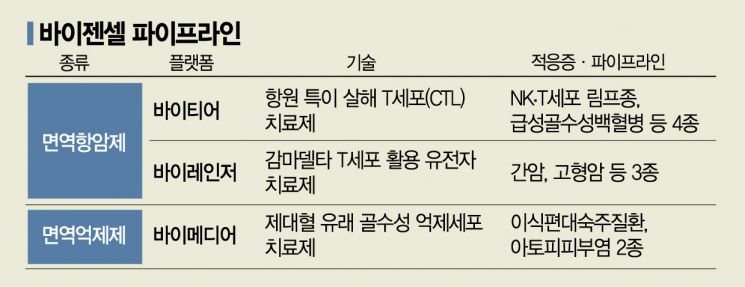
VigenCell develops both immuno-oncology drugs used in immunosuppressed states and immunosuppressants used in hyperimmune conditions. The company has three major platforms and nine pipelines: 'V-Tier (VT)' using antigen-specific cytotoxic T lymphocytes (CTLs) as immuno-oncology agents, 'V-Ranger (VR)' based on gamma delta T cells, and 'V-Medier (VM)' as an immunosuppressant.
The most advanced in clinical trials is 'VT-EBV-N,' indicated for NK/T-cell lymphoma, currently in phase 2. It works by culturing T cells collected from blood into antigen-specific cytotoxic T cells that target and eliminate cancer cells. CEO Kim said, "VT-EBV-N was designated as an orphan drug in the development stage by the Ministry of Food and Drug Safety in 2019. After completing phase 2, it can be marketed while conducting phase 3. We expect this to be possible around 2025."
V-Ranger uses gamma delta T cells, which do not cause allogeneic immune responses. Therefore, it has broader potential use compared to CAR-T therapies that require autologous production. Kim explained, "Gamma delta T cells have slightly less specificity than conventional T cells but do not attack other people's bodies, making them suitable for universal use and mass production. This complements the high cost and productivity limitations of autologous CAR-T therapies."
VigenCell is also developing 'VR-CAR,' which combines CAR technology with gamma delta T cells. Although no gamma delta T cell therapies have been commercialized yet, recent interim clinical results announced by U.S. company Addiset Bio showed complete remission with disappearance of cancer cells, raising expectations. He explained, "Conventional CAR-T therapies are mainly developed for blood cancers, but gamma delta T cells have high tissue penetration ability, allowing them to target solid tumors as well."
Developing Immunosuppressants for Immune Harmony
The immunosuppressive cell therapy platform V-Medier, indicated for atopic dermatitis and others, utilizes myeloid-derived suppressor cells extracted from umbilical cord blood. VigenCell is the first in the world to receive approval for human clinical trials of a universal immunosuppressive cell therapy using large-scale production technology of myeloid-derived suppressor cells. On the 9th of this month, preclinical results of 'VM-AD,' being developed as an atopic dermatitis treatment, were presented at the American Association of Immunologists.
In the third quarter of this year, an IND application for phase 1 clinical trials of VM-AD is planned in Australia. CEO Kim said, "Currently, the trend in cell therapies emphasizes anticancer immunotherapies, but even the atopic dermatitis market alone is as large as certain tumor markets. V-Medier and V-Ranger will gather objective data through overseas clinical trials and aim for licensing out."
Last month, VigenCell’s GMP facility was completed in Geumcheon-gu, Seoul. It is expected to obtain the necessary approvals for biopharmaceutical manufacturing and begin full-scale operations in the second half of this year. He said, "With the completion of the GMP facility, we now have the infrastructure to properly utilize pipelines that were previously delayed. All systems, including laboratory expansion, will be organized in the first half, so we will be ready to take off starting in the second half."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.