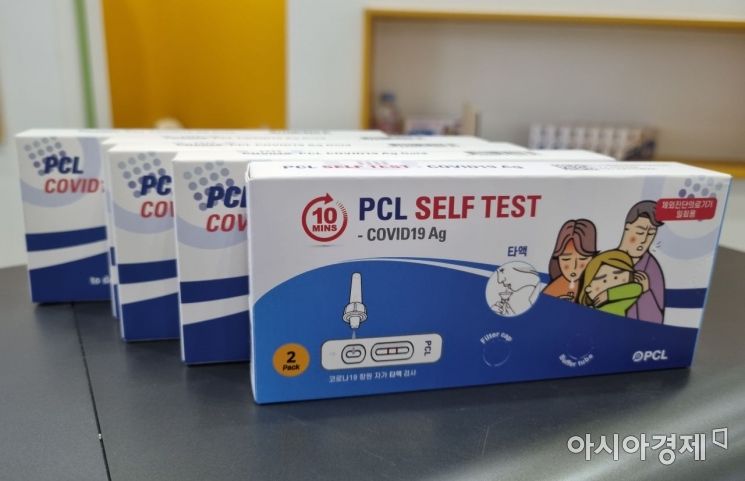
[Asia Economy Reporter Kim Young-won] PCL, a company specializing in in vitro diagnostics, announced on the 16th that it participated in Bio Korea 2022 held from the 11th to the 13th and showcased a saliva-based COVID-19 self-test kit.
At this event, PCL introduced products including the saliva-based COVID-19 test kit 'PCL Self-Test COVID19 Ag,' which received the first official domestic approval on the 29th of last month, and 'PCLOK II,' capable of rapid diagnosis of various diseases including infectious diseases. Bio Korea 2022 is the largest pharmaceutical and bio event in Korea, celebrating its 17th edition this year with participation from over 700 companies across 50 countries worldwide.
According to PCL, when the ABC cartridge is installed in the PCLOK II, it can simultaneously distinguish COVID-19, influenza A, and influenza B within 30 minutes. Its sensitivity is equivalent to that of large hospital equipment, allowing immediate testing and prescription at emergency rooms or primary care clinics. When equipped with the PCLOK II SARS-CoV-2 Dual IgG cartridge, it can verify past COVID-19 infection history and measure the antibody levels for the currently administered COVID-19 vaccine.
A PCL representative managing the booth stated, "We are sensing a lot of interest in the saliva self-test kit on-site," adding, "The product began selling at pharmacies and convenience stores starting this week, and detailed discussions for various distribution channels and supply are underway."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.