Biogen's 'Aduhelm'
Failed Commercialization but
Set Treatment Standards in the Market
Biogen Accelerates
FDA Approval Application for New Drug
Domestic Firms GemVax and Aribio
Rush Clinical Trials and Development
[Asia Economy Reporter Chunhee Lee] The world's first dementia treatment drug, Aduhelm (generic name Aducanumab), gained attention following approval by the U.S. Food and Drug Administration (FDA), but it is facing setbacks in actual commercialization. However, the pharmaceutical industry views the growing interest and potential in the dementia treatment market as an opportunity to secure market leadership and is actively pursuing follow-up development.
Failed Aduhelm Becomes a Global Standard
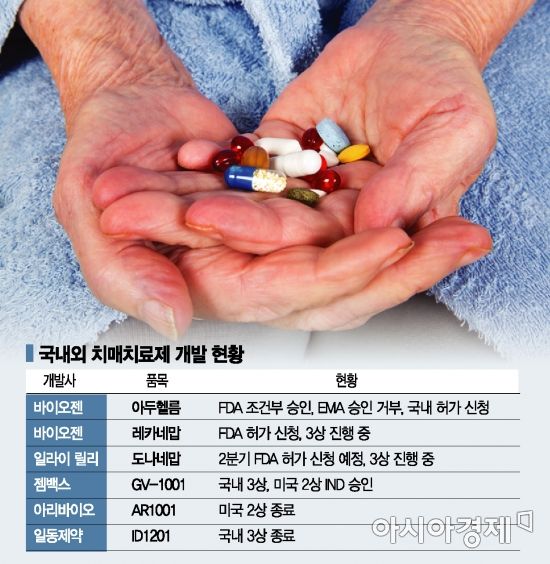
Aduhelm is an Alzheimer's treatment jointly developed by Biogen and Eisai. Although it received conditional approval for Phase 4 clinical trials in June last year, it was the first dementia treatment drug ever approved by the FDA. However, Aduhelm suffered a critical blow when the European Medicines Agency (EMA) rejected its approval in December last year. The EMA raised concerns that while Aduhelm reduces amyloid, it was not established whether this led to actual clinical improvement, and also pointed out the side effect known as ARIA (Amyloid-Related Imaging Abnormalities). Subsequently, the Centers for Medicare & Medicaid Services (CMS), which decides on U.S. insurance coverage, ruled that insurance would only apply to clinical trial participants, effectively deeming commercialization impossible.
There is also an observation that Aduhelm's "half-success" could actually open up the dementia treatment market. Mook In-hee, Director of the Dementia Overcoming Research and Development Industry Group, explained, "(The rejection of Aduhelm's approval) could actually be good news," adding, "Until now, there was no 'gold standard' for comparison, but now, only treatments showing better effects than Aduhelm must be approved, so a gold standard has been established." The argument is that if a treatment can prove effects exceeding Aduhelm's 23% clinical improvement, it could aim for FDA approval and market leadership, thus opening a new chapter rather than marking the end of the dementia treatment market.
Overseas, competition to secure the position of the next successor is intensifying. As of May last year, 126 candidate substances have been identified worldwide, with 152 clinical trials underway. Among these, 17 have entered Phase 3 clinical trials. In South Korea alone, the estimated number of dementia patients aged 65 and over is expected to reach 1.08 million by 2025 and 3.02 million by 2050.
The Cause of Dementia Remains 'Unclear'... Growth Potential Increases
Moreover, the fact that the cause of dementia has not been clearly identified is another reason for the growth potential of the dementia treatment market. It has been widely believed that removing proteins such as amyloid beta plaques, which increase in the brain as symptoms worsen, or tau proteins that misfold and tangle, would improve dementia symptoms. However, with Aduhelm, which targets amyloid beta, facing setbacks, demand for other mechanisms is gradually increasing. In fact, various attempts to develop treatments targeting tau proteins, synapses, and immunity, in addition to amyloid beta, are ongoing.
Biogen, the developer of Aduhelm, is once again leading the competition. On the 9th (local time), Biogen applied to the FDA for a Biologics License Application (BLA) for "Lecanemab," developed jointly with Eisai. Meanwhile, CEO Michelle Bonazos has stepped down, and the company has announced plans to continuously reduce the Aduhelm commercialization infrastructure, effectively abandoning the Aduhelm business. Eli Lilly recently revealed plans to apply for accelerated FDA approval for the Alzheimer's treatment "Donanemab" within the second quarter, aiming to complete FDA approval by next year.
In South Korea, various companies such as GemVax and Aribio are also developing dementia treatments. GemVax is currently preparing to start clinical trials after obtaining approval for Phase 3 in Korea and Phase 2 in the U.S. for "GV1001." GV1001 targets the fundamental cause of neuroinflammation by inducing antioxidant and anti-aging effects in brain nerve cells, viewing amyloid beta and tau proteins as symptoms. Aribio, having successfully completed Phase 2 clinical trials in the U.S. for "AR1001" last year, plans to proceed with Phase 3 trials based on this. Ildong Pharmaceutical has also completed Phase 3 clinical trials for a natural product-based dementia drug.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















