[Asia Economy Reporter Chunhee Lee] GemVax's clinical trials for 'GV1001,' a new drug under development for Alzheimer's treatment, will officially begin domestically and internationally this year.
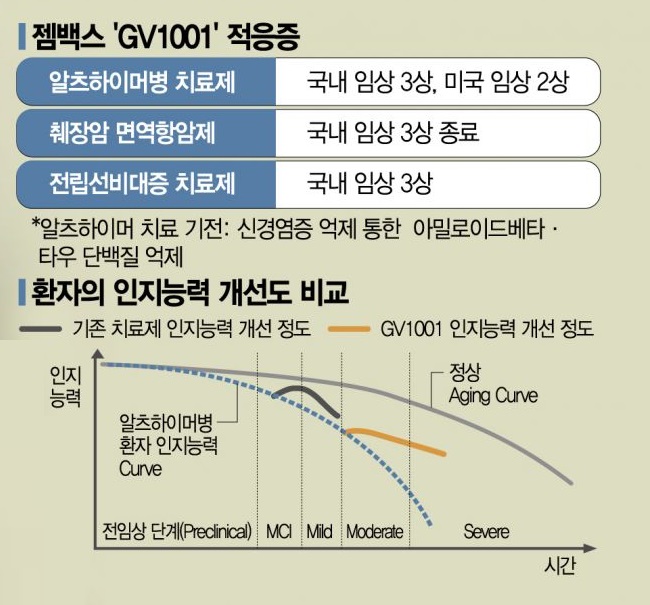
GemVax announced on the 9th that it plans to conduct Phase 3 clinical trials in Korea and Phase 2 trials in the United States for GV1001 this year. The Phase 3 trial, approved earlier this year, will be conducted at about 50 institutions in Korea, targeting 936 patients with moderate to severe Alzheimer's disease. The selection of the clinical research organization (CRO) and the principal investigator is currently being finalized. The company explained, "Among domestically developed new drugs, except for natural products, this is the first time a Phase 3 clinical trial for Alzheimer's is being initiated, and given its large scale, we are carefully selecting related parties."
The primary efficacy endpoints for the domestic Phase 3 trial are the changes in Severe Impairment Battery (SIB) and Clinical Dementia Rating-Sum of Boxes (CDR-SOB) scores after 24 weeks of GV1001 administration compared to baseline. The trial consists of a 24-week double-blind period followed by a 24-week open-label extension period.
The Phase 2 clinical trial in the United States will be conducted over 52 weeks at more than 40 institutions, targeting 180 patients with mild to severe Alzheimer's disease. The primary endpoint is set as the Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-cog11), which is widely used in mild to moderate clinical trials. The company added that Professor Martin Farrow, a world-renowned expert in dementia treatment from Indiana State University’s Department of Neurology, has been appointed as the principal investigator for this U.S. trial.
Meanwhile, GemVax is also pursuing the acquisition of treasury shares by executives and the company to enhance corporate value. Recently, through a public disclosure, the company decided to acquire 300,000 treasury shares worth 4 billion KRW and has currently acquired about 160,000 shares, nearly half of the planned amount.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.