[Asia Economy Reporter Lee Seon-ae] Kyobo Securities stated on the 27th that the key points to watch regarding Wontec's corporate presentation held for domestic and international investors are the sales volume of Ollijio and the timing of China's regulatory approval.
Wontec held a corporate presentation for domestic and international investors on the 24th. Established in 1999, Wontec is a beauty medical device company listed on the KONEX market in 2015. It is scheduled to be listed on the KOSDAQ market through a merger with Daishin Balance No.8 SPAC on June 30.
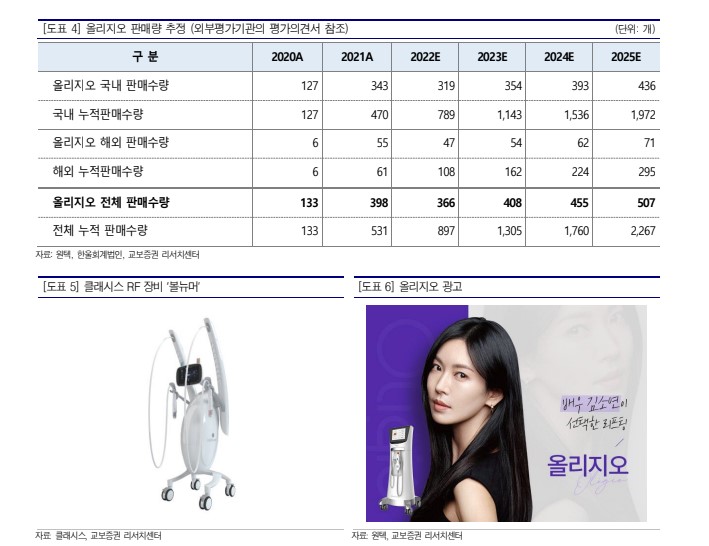
The main products include the laser device 'PicoCare', RF device 'Ollijio', and HIFU device 'Titan'. Thanks to the strong sales of the flagship product 'Ollijio', launched in 2020, last year's performance recorded sales of 51.1 billion KRW and operating profit of 10.4 billion KRW. Sales increased by 70.1% compared to the previous year, and operating profit turned positive.
In the beauty medical device industry, where selling more than 200 units is considered significant and exceeding 1,000 units earns the title of blockbuster product, Ollijio's sales volume of 630 units over about two years is a very encouraging figure. In the monopolar RF device market dominated by 'Thermage' and 'Ollijio', Ollijio quickly penetrated the market with equipment and consumable prices less than half of competing brands and satisfactory effectiveness.
However, due to the success of Ollijio and the increasing preference for non-invasive beauty procedures, domestic beauty medical device companies are planning to develop and launch new non-invasive RF devices one after another. Major products include Classys' 'Voluumer', JC's Medical 'next RF' (brand name undisclosed), Iruda's 'Thor', and Tentec (unlisted) 'TenTherma'.
Researcher Park Sung-guk of Kyobo Securities explained, "The key factor will be how much Ollijio's sales volume can be maintained at a high level in competition with upcoming non-invasive RF devices, thanks to the accumulated brand awareness through successful marketing with actress Kim So-yeon and the first-mover advantage from the 630 units already installed." He added, "It is necessary to observe the initial reactions to competing devices, marketing strategies, resources, and efficacy."
Despite China being the world's third-largest beauty medical market after the U.S. and Brazil, entry by domestic and foreign companies is difficult due to regulatory approval issues. To sell medical devices in China, additional clinical trials on Chinese nationals are required, and the approval process takes more than 2 to 3 years. During this period, companies must use overseas regulatory consulting firms, resulting in significant costs and time consumption.
However, Wontec is handling the Chinese medical device approval process in-house without outsourcing to a CRO company. Currently, it has obtained 15 NMPA (CFDA) approvals, demonstrating expertise in Chinese regulatory approval compared to other domestic beauty medical device companies. Regarding this, Researcher Park said, "No one currently knows the exact timing of Ollijio's approval, but based on experience, the company expects it to be around late 2022 to early 2023, which cautiously raises expectations."
China has not yet approved HIFU devices, and only some RF devices have received approval. Among them, one of the most popular procedures among Chinese people is Solta Medical's 'Thermage'. The approval of Ollijio's medical device in China is expected to expand exports. Researcher Park emphasized, "It is important to pay attention to the timing of regulatory approval in China in the future."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.