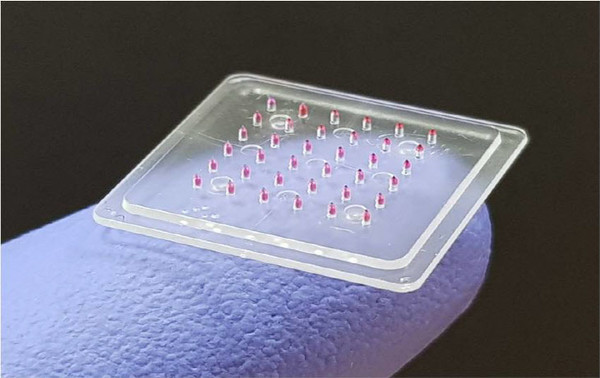
One-Third Hair Micro-Needle
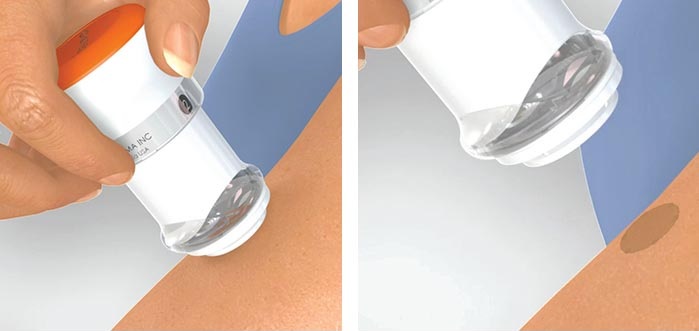
Patch Form Absorbed into the Body
No Pain or Burden Compared to Injections
Better Active Ingredient Absorption Than Oral Medication
Active Development and Investment by Domestic Companies
[Asia Economy Reporter Chunhee Lee] The ‘microneedle’ technology, which allows drugs that previously had to be injected or taken orally to be simply applied like a patch, is gaining attention.
Microneedles are a transdermal drug delivery system (DDS) that utilizes micro-needles about one-third the thickness of a human hair. They are typically used in patch form, applied to the skin for absorption into the body. Compared to injections, they cause less pain and burden, and unlike oral medications, they bypass liver metabolism processes, resulting in higher absorption of active ingredients and bioavailability.
Everyone Challenges Themselves to Develop the Technology
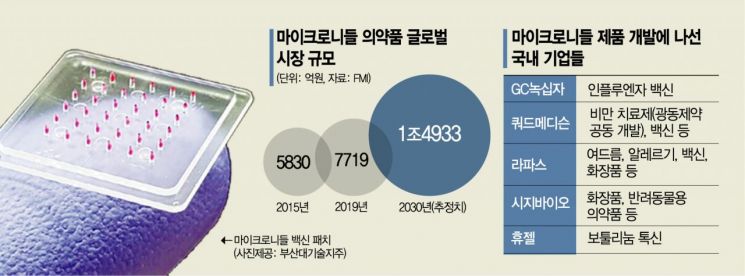
According to Future Market Insights (FMI) on the 20th, the global market size for microneedle pharmaceuticals is estimated to grow rapidly from $621.6 million (approximately 770.5 billion KRW) in 2019 to $1.2039 billion (approximately 1.4922 trillion KRW) by 2030. This is why investments and partnerships to secure related technologies are becoming active domestically as well.
Recently, CJ Bio made a paid-in capital investment in Daewoong Therapeutics, a venture subsidiary specializing in DDS-based new drug development under Daewoong, which owns a related microneedle platform. Through this, CJ Bio secured a 12.3% stake, and CJ Bio CEO Hyunseung Yoo was appointed as an inside director of Daewoong Therapeutics, directly participating in management. Previously, Daewoong Therapeutics and CJ Bio signed a microneedle technology transfer agreement.
Kwangdong Pharmaceutical partnered with Quad Medicine, which possesses related platform technology, aiming to develop obesity treatments. Kwangdong Pharmaceutical made a strategic investment of 2 billion KRW in Quad Medicine and will engage in joint research and development (R&D), while securing the first right of refusal for exclusive commercialization rights. Quad Medicine holds core technologies such as ‘multi-coated microneedles’ and ‘immediate separation microneedles’ and is developing various products.
Following the COVID-19 pandemic, attempts to introduce microneedle technology into vaccines are also ongoing. UK-based Emerges is conducting Phase 1 clinical trials in Switzerland for its patch-type COVID-19 vaccine ‘PepGNP.’ Domestically, UbioLogics is also pursuing the development of a patch-type COVID-19 vaccine.
Various vaccine developments are underway beyond COVID-19. Especially for vaccines, unlike conventional vaccines that require refrigerated or frozen distribution (cold chain), microneedle technology eliminates the need for such cold chains, which is considered an advantage. GC Green Cross is collaborating with US-based Vaxxas Technologies to advance Phase 1 clinical trials of a patch-type influenza vaccine. This combines the existing GC Flu vaccine antigen with microneedle technology. Quad Medicine is also developing a patch-type pentavalent vaccine containing diphtheria, pertussis, hepatitis B, and others, supported by the Light Fund funded by the Bill & Melinda Gates Foundation. Lapas has started developing a patch for the quadrivalent hepatitis B and influenza vaccine.
High Commercialization Barriers: Who Will Cross First?
However, the fact that no microneedle pharmaceuticals have been commercially launched worldwide remains a major obstacle in technology development. The company progressing the fastest is US-based Zosano Pharma. After completing Phase 3 clinical trials for the migraine treatment ‘Qtrypta,’ it is challenging the US Food and Drug Administration (FDA) for product approval. However, it has received a Complete Response Letter (CRL) and is attempting a reapplication. While the convenience of simply applying a patch is high, developing technology that allows microneedles to penetrate the skin’s stratum corneum, which protects the body from external environments, and effectively absorb drugs into the body is considered challenging.
However, as a platform technology, microneedles are expected to have high scalability since they can be applied to various fields such as cosmetics, growth hormones, botulinum toxin, and other medical devices or aesthetic sectors. In other fields, market entry is imminent. CJ Bio plans to launch the microneedle cosmetic ‘Dermarizen,’ containing EGF protein with collagen regeneration effects, in the first half of this year. Hugel aims to apply for Phase 1 clinical trials this year for botulinum toxin developed using microneedles.
Jung Yuntaek, Director of the Pharmaceutical Industry Strategy Research Institute, stated, “Microneedles are a market where the technology to effectively deliver a certain amount of drugs is not yet perfected, so commercialization has not been achieved. However, since there is unmet demand from patients who have burdens with existing injection methods or have difficulty with regular, precise oral dosing due to cognitive impairments, if these issues can be improved and overcome, patient benefits can be greatly enhanced.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![From Hostess to Organ Seller to High Society... The Grotesque Scam of a "Human Counterfeit" Shaking the Korean Psyche [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















