[Asia Economy Reporter Lee Gwan-joo] All orders, including the manufacturing and sales suspension order and the product approval cancellation disposition issued by the Ministry of Food and Drug Safety (MFDS) regarding Hugel's botulinum toxin product 'Botulinum,' will be stayed until the outcome of the related lawsuit is determined.
According to industry sources on the 11th, on the 8th, the Supreme Court dismissed the rehearing petition filed by the Seoul Food and Drug Safety Administration against the Seoul High Court's decision to cancel the product approval cancellation disposition for Botulax Injection.
Accordingly, both administrative dispositions imposed by the MFDS on Botulax Injection will be suspended until 30 days after the judgment date of the main lawsuit filed by Hugel against the Seoul Food and Drug Safety Administration.
The Supreme Court stated in its ruling, “After reviewing the lower court's decision and the reasons for the rehearing, it is clear that this rehearing falls under Articles 7 and 4 of the Special Act on the Supreme Court Procedure and is without merit; therefore, pursuant to Article 5 of the same law, the rehearing is dismissed.”
On the 5th, the Supreme Court also ruled in favor of Hugel regarding the 'provisional manufacturing and sales suspension order' for Hugel's Botulax Injection and dismissed the rehearing petition filed by the Seoul Food and Drug Safety Administration.
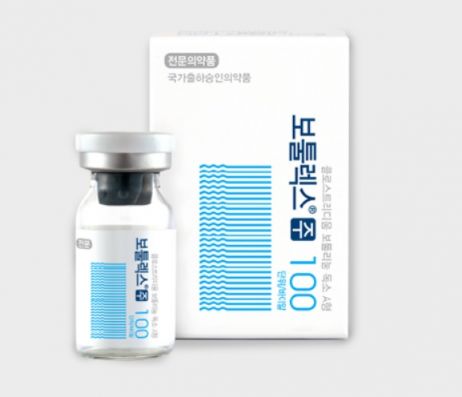
In December of last year, the MFDS, through the Central Investigation Unit for Serious Violations, detected that Hugel and PharmaResearch Bio sold six botulinum toxin products domestically without obtaining national batch release approval. Consequently, administrative actions including product approval cancellation, manufacturing and sales suspension, and recall and disposal procedures were initiated. Hugel responded with legal actions such as applying for a stay of execution.
A Hugel representative stated, “The products subject to the MFDS's disposition were export-only pharmaceuticals produced and sold for export purposes and are not subject to national batch release approval.” He added, “We have faithfully complied with the MFDS guidelines and the Foreign Trade Act, which state that national batch release approval is not required for exports, and there was absolutely no intention to evade or circumvent the regulations.”
He continued, “Our company is on the verge of a true global leap, with expected entry into the North American and Oceania toxin markets this year following China and Europe,” and added, “We will focus our capabilities to ensure that the corporate value Hugel has diligently built is not shaken through the upcoming main lawsuit.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
!["The Woman Who Threw Herself into the Water Clutching a Stolen Dior Bag"...A Grotesque Success Story That Shakes the Korean Psyche [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















