Cell Therapy Made from Patient's Cells
High Cost Due to 'Custom Production'
Need to Develop 'Allogeneic' for Mass Production
[Asia Economy Reporter Chunhee Lee] From this month, Novartis' "Kymriah," known as an "ultra-high-cost anticancer drug," has been included in the health insurance coverage. The domestic reimbursement ceiling for a single administration is set at 360.04 million KRW, and it is known as an ultra-high-cost anticancer drug costing over 500 million KRW per administration in the United States. However, since the application of health insurance from the 1st of this month, the cost burden for patients has been reduced to about 6 million KRW. However, health insurance coverage is applied only once per patient for a lifetime.
It took nearly a year for Kymriah to be covered by health insurance. Typically, it takes about nine months from the pharmaceutical company's application to insurance coverage, but it took a considerable amount of time in this case. The delay was largely due to controversy over Kymriah's excessive cost. Although it is highly effective enough to almost completely cure patients with acute lymphoblastic leukemia and lymphoma with just one administration, earning it the nickname "one-shot anticancer drug," there was debate over whether it is appropriate for the state to pay several hundred million KRW in health insurance benefits to an individual at once. During last year's national audit, there were also calls to include Kymriah in health insurance coverage quickly.
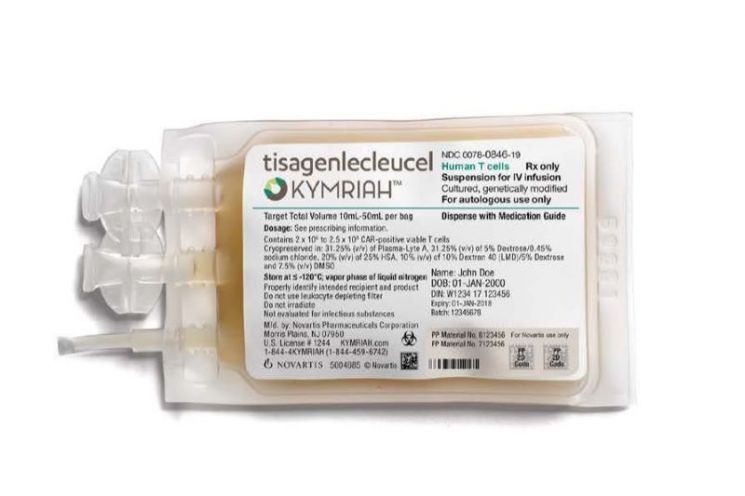
Why is Kymriah so expensive? Kymriah is a cell therapy among the recently spotlighted gene and cell therapies (CGT). It is the world's first chimeric antigen receptor T-cell (CAR-T) therapy. Cells from patients suffering from leukemia are taken to Novartis' factory in the United States, where it takes about a month to produce the cell therapy. The therapy works by introducing genetic information into the patient's immune cells so that they can recognize specific antigens on cancer cells and attack them. Unlike general anticancer drugs, the manufacturing method, efficacy, and dosage are all completely different. Since the therapy is made based on the patient's own immune cells, it is a thoroughly personalized treatment. Because mass production is impossible, the price inevitably skyrockets.
On the other hand, the effectiveness is clear. In studies targeting patients with acute lymphoblastic leukemia, as many as 82% of patients achieved complete remission within three months. Actual clinical statistics also show that more than half of the patients survive for over two years.
The problem arises when such personalized CGT therapies are released one after another. Since they are made by directly intervening in an individual's genes or cells, the effectiveness is excellent, but mass production is impossible, so prices inevitably rise. Given that the number of patients who can actually receive treatment is limited, this is why the global market size for CAR-T therapies remains at around 1 trillion KRW.
The industry is continuing efforts to overcome this. There are calls to develop "allogeneic" CGT therapies, rather than autologous methods like Kymriah, to lower prices and expand the market. Representative examples include ALLO-715, a treatment for relapsed refractory multiple myeloma by the U.S. company Allogene, and UCART19, a treatment for acute lymphoblastic leukemia.
Such allogeneic CAR-T therapies use immune cells cultured from blood held in cell banks and can be administered to multiple patients, making them cheaper and allowing for rapid administration. However, since blood from others is injected, immune response management through immunosuppressants is necessary, which is a drawback.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















