Patient Burden Reduced from 400 Million KRW to 5.98 Million KRW
However, Applied Only Once Per Person
[Asia Economy Reporter Ki Ha-young] Starting next month, health insurance will cover the acute lymphoblastic leukemia treatment drug 'Kymriah,' reducing patient costs by up to 5.98 million KRW.
The Ministry of Health and Welfare announced on the 31st that it held a meeting of the Health Insurance Policy Deliberation Committee (HPC), the highest decision-making body for health insurance, and revised the drug benefit list and the benefit ceiling price table.
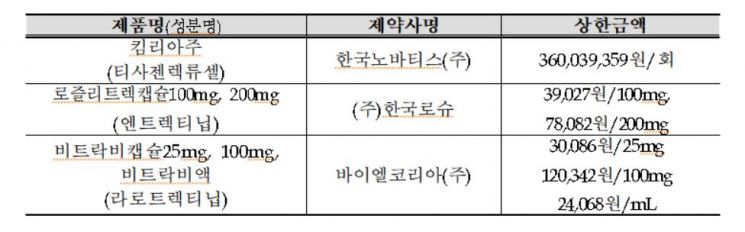
The HPC decided to apply health insurance coverage from the 1st of next month for Kymriah, an ultra-expensive gene therapy used to treat acute lymphoblastic leukemia and diffuse large B-cell lymphoma. Accordingly, the current single-dose cost of Kymriah, which is about 400 million KRW, will be reduced to a patient burden of up to 5.98 million KRW (applying the health insurance out-of-pocket ceiling system). However, health insurance coverage is limited to one lifetime dose per patient.
Additionally, health insurance will also cover medical procedures such as cell collection and drug infusion performed during the administration of CAR (Chimeric Antigen Receptor) T-cell therapies like Kymriah. The fees were newly established by referencing the stepwise treatment costs of hematopoietic stem cell transplantation. With health insurance coverage, patient medical expenses excluding the cost of CAR T-cell therapy drugs will decrease from 2 to 4 million KRW to about 100,000 KRW (based on a 5% co-payment rate).
Furthermore, the HPC decided to apply health insurance coverage from next month for Rozlytrek capsules (100 mg, 200 mg), Vitrakvi capsules (25 mg, 100 mg), and Vitrakvi solution, which are NTRK gene fusion-positive solid tumor treatments. Rozlytrek capsules have an annual treatment cost of about 85 million KRW, but with health insurance coverage, patient costs will be reduced to approximately 4.3 million KRW. The annual treatment cost of Vitrakvi capsules and solution, which was about 88 million KRW, will also be reduced to around 4.4 million KRW for patients.
At this HPC meeting, changes to health insurance benefits were also discussed for four selective benefit items: ▲NK cell activity test (precision immunoassay), ▲blood perfusion therapy using polymyxin B immobilized fiber, ▲sutureless aortic valve replacement, and ▲transcatheter aortic valve implantation. The changes will take effect from May 1.
First, regarding the NK cell activity test (precision immunoassay), the suitability assessment showed unclear test efficacy, and literature reviews indicated insufficient evidence for its application in some cancer patients. However, the HPC judged that it is better to keep this test within the benefit system to manage potential misuse. Therefore, the test will remain a selective benefit, but the patient co-payment rate will increase from 80% to 90%, and criteria such as eligible patients and the number of tests will be set to minimize unnecessary use.
Blood perfusion therapy using polymyxin B immobilized fiber, which was evaluated as an ineffective technology, will be converted to non-benefit coverage. For sutureless aortic valve replacement, benefit criteria will be established to apply coverage mainly to cases with high clinical necessity, while other cases will have a 50% patient co-payment rate.
Regarding transcatheter aortic valve implantation, coverage will be applied to those aged 80 and above, while for other age groups, coverage or selective benefit will be applied differently depending on surgical risk. The HPC plans to conduct additional reviews on coverage eligibility for this item around next year when domestic research is completed.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.