Theory of 'Carbon Cap Protrusion Phenomenon' on Catalyst Particle Surface Elucidated... Published in JACS
Expected to Aid Development of CNT Synthesis Catalysts Noted as Battery Additives
[Asia Economy Yeongnam Reporting Headquarters Reporter Dongguk Lee] 'Carbon nanotubes' grow by rising from the catalyst surface. It seems to defy gravity.
UNIST researchers have revealed the secret of this growth for the first time. It has been 30 years since carbon nanotubes were discovered.
The research team led by Professor Feng Ding of the Department of Materials Science and Engineering at UNIST published a new theory explaining the essential step of carbon nanotube synthesis, the 'cap rising' phenomenon, in the Journal of the American Chemical Society (JACS).
The team stated, "We were able to uncover a phenomenon overlooked for the past 30 years through molecular simulations and density functional theory analysis," and added, "This will help in designing catalyst structures for synthesizing carbon nanotubes."
Carbon nanotubes have excellent conductivity, strength, and ductility, and since their discovery in 1991, they have been one of the most studied carbon materials alongside graphene (a paper-thin material where carbon atoms are connected in a honeycomb pattern).
They are also attracting attention as a material that could replace silicon, which is used in memory semiconductors.
Although this material looks like graphene rolled up, it actually grows vertically on the catalyst surface and is synthesized in the form of a hollow cylinder.
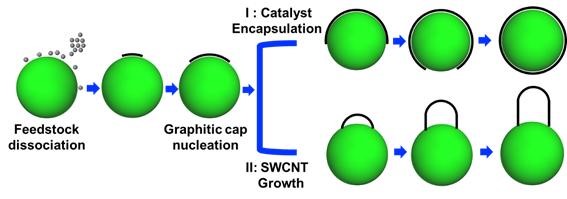
'Cap rising' is a phenomenon where the cap structure formed by carbon gathering at the catalyst's edge rises in a dome shape.
It occurs at the early stage of the synthesis reaction. Without this cap rising phenomenon, the reaction would end with carbon atoms and catalyst particles completely surrounding the spherical catalyst surface.
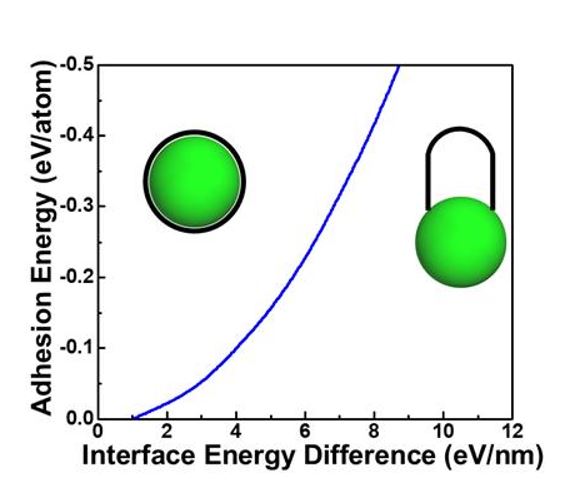
According to Professor Ding's team, the cause of this phenomenon is the reduction of interfacial energy between the cap edge and the catalyst surface. The energy reduction offsets the attractive force between the catalyst and carbon atoms, causing the cap structure to spontaneously lift up.
Simulation results confirmed that as the cap lifts, that is, as the angle between the cap wall and the catalyst surface approaches 90 degrees, the interfacial energy decreases.
Since the interfacial energy is affected by the contact angle formed between the cap edge and the catalyst particle, the research team explained that the size of the contact angle can predict whether the cap structure will rise.
According to the prediction results, when the contact angle is between 45 and 90 degrees, the cap structure rises well and the carbon nanotube synthesis reaction proceeds effectively, which aligns with existing experimental results.
This study was published on the 17th in the Journal of the American Chemical Society, a prestigious journal in the field of chemistry.
Researchers LiPing Ding and Ben McLean from the IBS Center for Multidimensional Carbon Materials, and Ziwei Xu from Jiangsu University in China participated as co-first authors. The research was supported by IBS (Institute for Basic Science) and others.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
!["The Woman Who Threw Herself into the Water Clutching a Stolen Dior Bag"...A Grotesque Success Story That Shakes the Korean Psyche [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















