Interview with Song Hyung-gon, CEO of GemVax
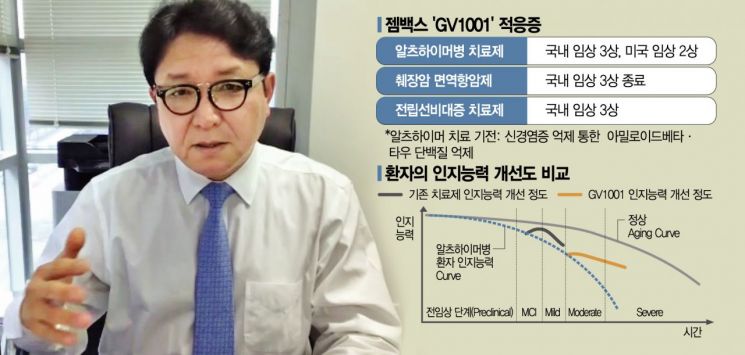
Development of Alzheimer's Treatment 'GV1001'
Recently Approved for Phase 3 Clinical Trial in Korea
Phase 2 Trials Also Planned in the US and Europe
Aiming to Inhibit Progression at the 'Severe' Stage
Also Shows Positive Effects on Pancreatic Cancer and Benign Prostatic Hyperplasia
Additional Clinical Trials Underway
[Asia Economy Reporter Lee Chun-hee] "GV1001 is a multi-target therapeutic that reduces amyloid beta protein and prevents tau protein tangling by suppressing neuroinflammation. After the domestic Phase 3 clinical trial, we will steadily proceed with Phase 2 trials in the US and Europe." (Song Hyung-gon, CEO of GemVax)
The exact cause of Alzheimer's dementia is still unknown. It has been widely believed that removing these proteins could improve dementia symptoms, as the amyloid beta protein in the patient's brain turns into plaques and increases, or tau protein misfolds and tangles as symptoms worsen.
However, Biogen's Aduhelm, developed based on the amyloid beta mechanism and approved by the US Food and Drug Administration (FDA) in June last year, has only achieved partial success as it struggles to expand its market. This is why the global Alzheimer's treatment market, expected to grow to about 20 trillion KRW within a few years, remains largely untapped.
GV1001, developed by GemVax as an Alzheimer's dementia treatment, takes a somewhat different approach. Since amyloid beta and tau proteins are symptoms rather than causes, it aims to eliminate the fundamental cause?neuroinflammation?by inducing antioxidant and anti-aging effects in brain nerve cells.
In an interview with Asia Economy on the 11th, CEO Song Hyung-gon explained, "Ultimately, the basic unit of life is the cell. The body can only be healthy if the cells are healthy, and if there is a problem with the cells, various diseases can occur."
CEO Song also expressed strong confidence in the recently approved domestic Phase 3 clinical trial. He said, "Among those who participated in the emergency Phase 2 trial, some felt the effects and wanted to continue the treatment, and the participating professors frequently inquired about when Phase 3 would start, as there were symptom improvements."
The approval process for GV1001's Phase 3 clinical trial was not smooth. After completing Phase 2 in 2019, the Phase 3 application was submitted in January last year but was rejected and required supplementation before finally being approved in January this year. During this process, the Central Pharmaceutical Review Committee, usually convened at the new drug approval stage, issued an advisory recognizing the limited therapeutic use of GV1001. CEO Song noted, "It is unusual for the Central Pharmaceutical Review Committee to be involved in clinical trial approval rather than new drug approval," but emphasized, "Since this is a globally noteworthy and still unexplored area, it is appropriate to review it step by step."
GemVax is currently proceeding with related procedures such as seeking a Contract Research Organization (CRO) for clinical trial progress. They are also actively promoting global clinical trials. He said, "In addition to the US Phase 2 trial, we plan to sequentially apply for Phase 2 trials with the European Medicines Agency (EMA)."
 GemVax's 'GV1001'
GemVax's 'GV1001'
Regarding some claims that GV1001 does not lead to a 'complete cure,' CEO Song explained, "It depends on how you view the concept of a cure. The treatment goal is to suppress progression at the moderate stage or higher and prevent further deterioration." He added that GV1001 aims to prevent patients from progressing to the 'severe' stage, where cognitive decline due to dementia requires 24-hour care and hospitalization in nursing hospitals.
On GV1001's indications beyond Alzheimer's, such as pancreatic cancer and benign prostatic hyperplasia, he stated, "Because it shows beneficial effects inside cells." During the development of a pancreatic cancer anticancer drug, incidental benefits such as cognitive improvement and prostate size reduction were discovered, leading to clinical trials to add these as indications.
However, CEO Song noted, "There are other inflammation-related data, but considering the company's size, the current indication level is appropriate for now." He added, "The Phase 3 domestic clinical trial for the pancreatic cancer anticancer drug has been completed, and if the paper is published within this year, we plan to apply for approval accordingly. The benign prostatic hyperplasia treatment trial will also conclude in mid-month, with results expected to be announced within this year."
GemVax is developing various pipelines beyond GV1001. At the core is 'peptides.' Peptides are substances composed of fewer than 50 amino acids, known for high tissue penetration and multifunctional effects, attracting attention as next-generation drugs. CEO Song said, "We hold more than 200 patents related to peptides domestically and internationally," and added, "Based on the success of GV1001, we will develop various new drugs including antiviral and brain disease treatments."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.