Oxygen Saturation Below 95% Raises Hypoxia Concerns
Blocking Light Is Important for Accurate Measurement
[Asia Economy Reporter Ki Ha-young] As the Omicron variant spreads and the number of COVID-19 patients receiving home treatment approaches 1 million, interest in 'pulse oximeters,' devices used to measure blood oxygen saturation, is also increasing. We have summarized the important points to be careful about for accurate measurement.
According to the Ministry of Food and Drug Safety on the 5th, the most important aspect when using a pulse oximeter is to block out light. Pulse oximeters generally measure the amount of infrared light (red) emitted from a light source that passes through or reflects off the finger and reaches the sensor to calculate the blood oxygen saturation.
Pulse Oximeters: Blocking Light is Important... Consult a Doctor if Below 95%
Therefore, if the measurement area of the device is exposed to bright surrounding light during pulse oximetry, the measurement may be inaccurate, so it is important to block out light as much as possible. Insert the index finger with the back of the hand facing upward and avoid moving while the device is measuring.
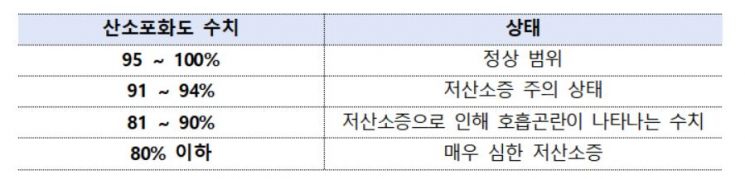
After the measurement, check the oxygen saturation level, and if it is below 95%, visit a medical institution and consult a doctor. A range of 95?100% is normal, 91?94% indicates a cautionary state of hypoxia, 81?90% is a level where breathing difficulties due to hypoxia appear, and below 80% is considered very severe hypoxia.
It is also necessary to maintain finger cleanliness before using the pulse oximeter. Polished nails, artificial nails, and nail polish reduce the transmission of infrared light, so it is recommended to remove them. If the pulse oximeter is not used for a long time, remove the battery and store it in a place not exposed to direct sunlight.
Medical Purpose Products Display Certification Number and Model Name on Packaging
When purchasing a pulse oximeter, it is important to select according to its intended use. Depending on the purpose, it can be classified as a medical device for diagnosis and treatment or as a general product for exercise and leisure purposes.
In particular, pulse oximeters used for medical purposes are classified as medical devices and are certified and managed by the Ministry of Food and Drug Safety. In this case, products must obtain manufacturing/import certification according to the Medical Device Act, and medical-purpose product packaging displays the medical device certification number and model name. Whether a product is certified by the Ministry of Food and Drug Safety can also be verified on the Medical Device Electronic Civil Service Portal website.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.