Pre-Booking Available from the 21st... Vaccination Dates from March 7 Onwards
Cross-Vaccination Allowed Upon Doctor's Discretion
[Asia Economy Reporter Ki Ha-young] Starting from the 14th, Novavax vaccine administration will begin for high-risk groups with a high risk of severe illness or death and unvaccinated adults aged 18 and over against COVID-19.
On the 10th, the COVID-19 Vaccination Response Promotion Team announced this vaccination implementation plan following the shipment of Novavax vaccine doses, which were consigned for production by SK Bioscience the previous day.
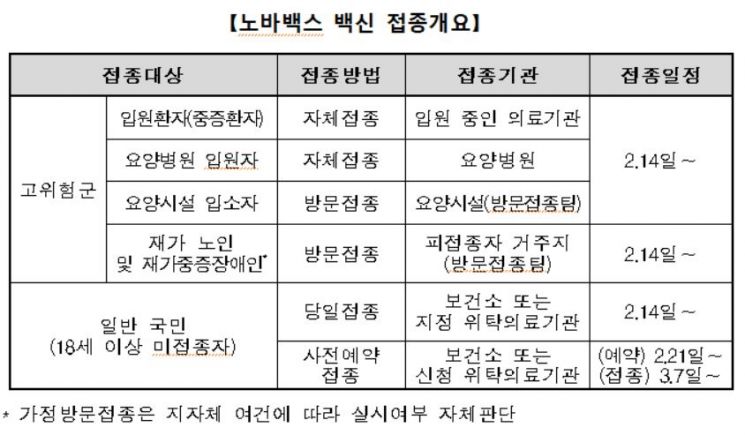
The team explained that the vaccination will first be carried out for basic vaccination (1st and 2nd doses) of adults aged 18 and over who have not yet received a COVID-19 vaccine, as well as for high-risk groups such as hospitalized patients at high risk of severe illness or death, homebound elderly, and severely disabled individuals.
Accordingly, from the 14th, vaccinations for high-risk groups will be conducted through in-hospital and facility-based self-administration and home visits. The general public can also receive same-day vaccinations at public health centers and designated medical institutions nationwide by reserving leftover vaccines or using medical institution standby lists via KakaoTalk and Naver starting on the same day.
From the 21st, advance reservations will be available through the vaccine pre-booking website, with vaccination dates selectable from March 7 onward.
Cross-vaccination Requires Physician’s Judgment
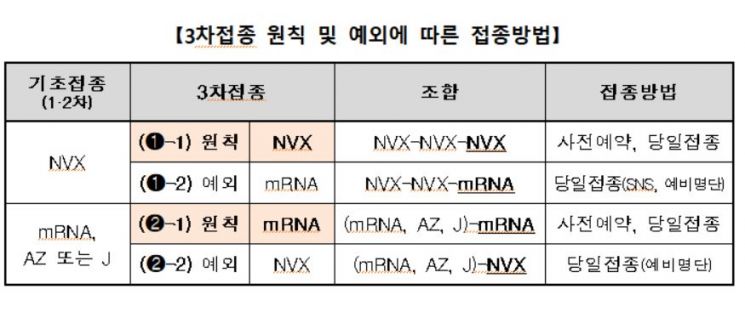
Novavax vaccine will also be used for the third dose. The basic principle is that if the primary vaccination (1st and 2nd doses) was conducted with the Novavax vaccine, the third dose will also be administered with Novavax. Both pre-booked and same-day vaccinations are possible, with the interval being at least three months after the second dose.
However, if a person who received the primary vaccination with Novavax wishes to receive the third dose with an mRNA vaccine, vaccination is possible even without special reasons. In this case, vaccination can only be done via same-day appointments, and pre-booking is not available.
Those who completed their primary vaccination with AstraZeneca (AZ), Pfizer, Moderna, or Janssen vaccines but have contraindications or postponement reasons for other vaccines can also receive the third dose with Novavax. Physician judgment is required in this case. Eligible individuals can register on the medical institution’s standby list and receive vaccination after completing same-day reservation.
The promotion team stated, "The Novavax vaccine is produced using a recombinant gene technology method, similar to hepatitis B and influenza vaccines," and added, "Since it is a vaccine with extensive vaccination experience among the public, we expect it to serve as an opportunity for unvaccinated citizens to participate more actively in vaccination."
Additional 294,000 Doses of Novavax Vaccine Scheduled for Shipment on the 11th
Meanwhile, 292,000 doses of the Novavax vaccine were shipped from SK Bioscience’s Andong plant the previous day, and an additional 551,000 doses were supplied on the same day. Another 294,000 doses are scheduled to be shipped on the 11th.
The government has secured a total of 150.44 million doses of COVID-19 vaccines this year through contracts with individual pharmaceutical companies and international organizations, as well as international cooperation, including Pfizer, Moderna, Janssen, and Novavax vaccines. As of the 11th, 6.35 million doses have been delivered. The government plans to procure an additional 144.09 million doses by the end of this year.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.