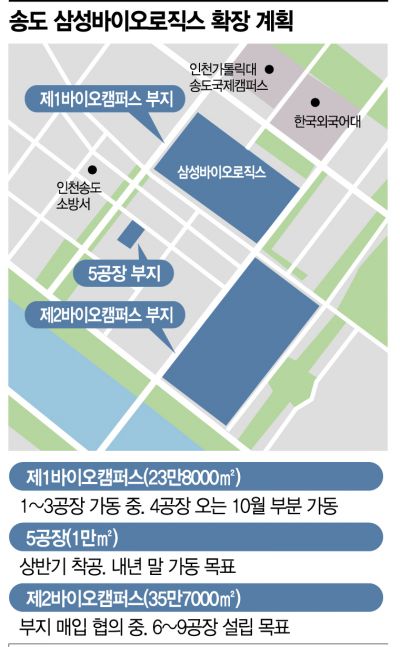
Additional Land Secured for Incheon Songdo 2nd Campus
Plant 5 to Become a Multi-Production Facility
[Asia Economy Reporter Lee Chun-hee] Samsung Biologics will partially operate its 4th plant in October and start construction of the 5th plant in the first half of the year to expand production capacity. It is also accelerating plans to establish the 6th to 9th plants by building a 2nd Bio Campus in Songdo, Incheon. Following its base in San Francisco, USA, it will secure a foothold in Boston on the East Coast and continuously strengthen capabilities by expanding contract manufacturing organization (CMO) for messenger ribonucleic acid (mRNA) active pharmaceutical ingredients (DS) and cell and gene therapy (CGT) products.
John Rim, CEO of Samsung Biologics, held an online press conference on the 13th, stating, "The 4th plant aims for partial operation by the end of the year, and we are strengthening pre-order activities. The 5th plant will also begin construction within the year to further expand our business portfolio." He added, "Currently, the 1st Bio Campus in Songdo is fully utilized, so we are securing additional land for the 2nd Bio Campus and consider the 6th to 9th plants as future industries."
The 1st Bio Campus of Samsung Biologics, located in Songdo, Incheon, covers an area of 278,000㎡ and has no remaining land after the 4th plant. To expand further, the company purchased about 10,000㎡ of land near the 1st campus for the 5th plant from the Incheon Free Economic Zone Authority in November last year and is currently negotiating with the Incheon Economic Free Zone Authority to acquire 357,366㎡ of land in Songdo District 11. Rim stated, "While constructing new facilities, we will also create an open innovation center to nurture bio ventures and a global R&D analysis facility to strengthen capabilities." Although still in the preliminary negotiation stage, there is little chance of unexpected changes in securing the land since no other buyers besides Samsung Biologics have shown interest.
Through this capacity expansion, Samsung Biologics plans to increase its current biopharmaceutical production capacity of 364,000 liters to 620,000 liters by the second quarter of next year when the 4th plant is completed. Rim explained, "The 4th plant has already signed contracts for five products with three top-tier biopharma companies and is discussing contracts for 30 products with 20 pharmaceutical companies."
Additionally, the company revealed a vision to expand its current business portfolio, which mainly focuses on antibody drug CMO, to include CGT and next-generation vaccine CMO based on mRNA, plasmid DNA (pDNA), and viral vectors. The 5th plant is key to this new business. Construction is scheduled to start in the first half of this year, with operation targeted by the end of next year. It will be built as a ‘Multi Modal’ plant capable of producing various types of biopharmaceuticals in a single facility, supporting the expansion of new businesses.
The mRNA vaccine production will extend beyond drug product (DP) manufacturing to include DS production. Previously, Samsung Biologics signed a clinical trial material CMO contract for an mRNA vaccine candidate developed by GreenLight Biosciences in the USA. The company plans to obtain cGMP approval by the second quarter of this year and start producing the vaccine from May. Currently, Samsung Biologics only handles DP production, such as filling and packaging Moderna’s mRNA vaccine. Rim said, "Moderna only requires DP production and has not requested DS production yet," adding, "We are continuously negotiating with other clients to secure more orders independently of Moderna."
 John Rim, CEO of Samsung Biologics, is answering questions at an online press conference held on the morning of the 13th. (Photo by Samsung Biologics)
John Rim, CEO of Samsung Biologics, is answering questions at an online press conference held on the morning of the 13th. (Photo by Samsung Biologics)
Samsung Biologics also plans to seek additional overseas bases. Following the opening of the contract development (CDO) R&D center in San Francisco on the US West Coast in 2020, it will secure a base in Boston on the East Coast and simultaneously review entry into Europe. However, regarding plant expansion, Rim added, "We are considering Korea because it costs much less and can be built faster."
When asked about mergers and acquisitions (M&A) to secure new business momentum, Rim responded, "We are considering M&A as well. We will comprehensively review direct expansion or M&A based on current market conditions and profitability." Last month, rumors surfaced about Samsung Group’s acquisition of Biogen, a US biotech company that co-owns Samsung Bioepis, a subsidiary of Samsung Biologics. However, Samsung Biologics strongly denied the rumors through an official disclosure.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.

![User Who Sold Erroneously Deposited Bitcoins to Repay Debt and Fund Entertainment... What Did the Supreme Court Decide in 2021? [Legal Issue Check]](https://cwcontent.asiae.co.kr/asiaresize/183/2026020910431234020_1770601391.png)














