Hyun Taek-hwan IBS Nanoparticle Division Joint Research Team Develops Photocatalyst
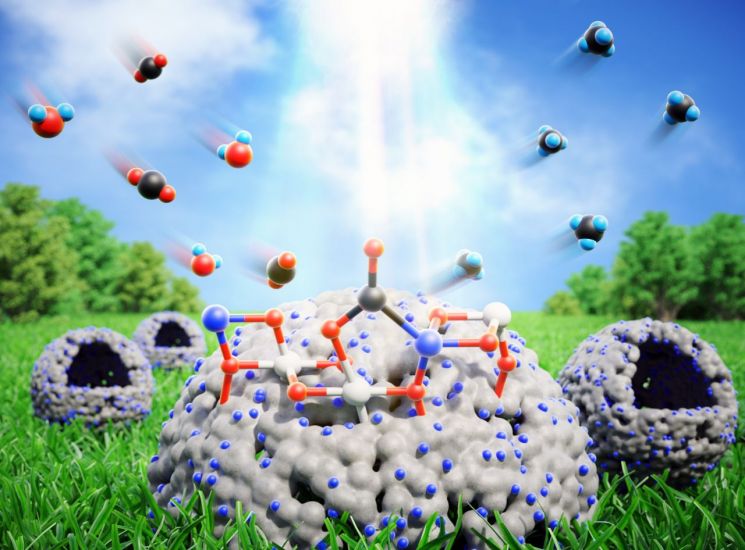 Titanium dioxide catalyst mechanism that converts carbon dioxide into hydrocarbons using only sunlight and water.
Titanium dioxide catalyst mechanism that converts carbon dioxide into hydrocarbons using only sunlight and water.
[Asia Economy Reporter Kim Bong-su] Domestic researchers have developed a new catalyst that can convert carbon dioxide into useful fuel using only water and light, without the addition of any separate compounds.
The Institute for Basic Science (IBS) announced that the research team led by Hyun Taek-hwan, head of the Nanoparticle Research Division and distinguished professor at Seoul National University, in collaboration with Professor In Soo-il's team at Daegu Gyeongbuk Institute of Science and Technology (DGIST) and Professor Kim Hyung-joon's team at Korea Advanced Institute of Science and Technology (KAIST), developed a photocatalyst that converts carbon dioxide into useful hydrocarbon energy such as methane and ethane, and elucidated its operating mechanism.
A photocatalyst is an eco-friendly system that can directly convert solar energy into chemical fuel. In previous research, the IBS Nanoparticle Research Division developed a single-atom copper/titanium dioxide (TiO2) catalyst by placing copper atoms on TiO2 nanoparticles, a photocatalyst, and succeeded in producing hydrogen using sunlight and water. The catalyst developed at that time attracted attention by improving catalytic performance by more than several tens of times without using expensive precious metals.
In this study, the team further clarified the mechanism by which the single-atom copper/TiO2 photocatalyst interacts with carbon dioxide at the atomic level, and based on this, succeeded in developing a catalyst that converts carbon dioxide into chemical fuel using only sunlight and water.
First, the researchers focused on the metal-support interaction occurring between stabilized copper atoms and the TiO2 photocatalyst. Metal-support interaction refers to the chemical interaction between the support and the metal catalyst placed on it. It is an essential factor to enhance catalytic activity, but precise atomic-level control has been difficult.
The researchers discovered that controlling the metal-support interaction at the atomic level creates active sites where carbon dioxide can be effectively converted, and based on this, designed a single-atom copper/TiO2 catalyst. This optimized photocatalyst was able to convert carbon dioxide into hydrocarbon fuels such as methane and ethane with an efficiency more than 60 times higher than conventional TiO2 photocatalysts.
Co-corresponding author Professor In Soo-il of DGIST said, “The results of this study can be broadly applied to improve the performance of not only TiO2 photocatalysts but also various other types of photocatalysts,” and added, “We plan to conduct follow-up research to apply the carbon dioxide photo-conversion mechanism to various photosynthetic catalysts.”
Division head Hyun Taek-hwan stated, “For a carbon-neutral society, it is necessary to reduce carbon usage while converting emitted carbon dioxide back into chemical fuel,” and added, “With further research, it may be possible to convert carbon dioxide into higher value-added chemicals such as ethanol and propanol using photocatalysts.”
The results of this study were published in the online edition dated September 20 in the academic journal Energy & Environmental Science (IF 38.532), a leading journal in the field of energy and environmental science.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![From Bar Hostess to Organ Seller to High Society... The Grotesque Con of a "Human Counterfeit" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















