Professor Hong Seok-won and Team at Gwangju Institute of Science and Technology
[Asia Economy Reporter Kim Bong-su] A Korean research team has developed the world's first catalyst that can stop a chemical reaction when exposed to light. Existing photo-switching catalysts all initiate reactions upon light exposure, but this study presents the first case where the reaction stops when exposed to light.
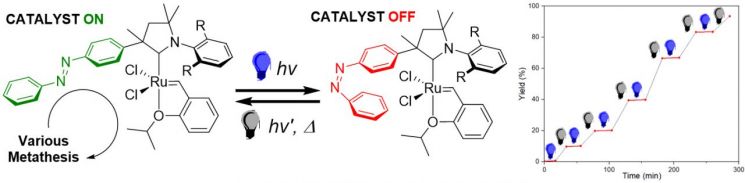
The research team led by Professor Hong Seok-won of the Department of Chemistry at Gwangju Institute of Science and Technology (GIST) announced on the 16th that they developed a ruthenium olefin metathesis catalyst whose reactivity can be controlled in real time using light.
Ruthenium is a platinum-group element, a metal that is hard yet brittle, and acts as a catalyst for hydrogenation and oxidation. Olefin metathesis is a reaction in which carbon-carbon double or triple bonds in starting materials are broken and reformed into new carbon-carbon double or triple bond compounds using metal catalysts such as ruthenium. It is used in the synthesis of cyclic and acyclic alkenes with various functional groups as well as polymers.
Enzymes, the best catalysts created by nature, can organically switch between active and inactive states in response to external stimuli. Accordingly, chemists have been challenging themselves to create functional catalysts that can switch states via external stimuli beyond simple catalysts. Olefin metathesis catalysts have been widely applied in synthesizing biologically active organic compounds such as polymers, fuel additives, and pharmaceuticals by enabling the formation of new bonds or the introduction of various functional groups. Recently, the development of catalysts capable of switching olefin metathesis reactions via external stimuli has been progressing. All existing photo-switching catalysts initiate the metathesis reaction upon light exposure. The switching catalyst developed by the research team is the first case where the reaction stops when exposed to light, contrary to previous catalysts.
The team focused on the azobenzene functional group (a molecule where two phenyl groups are connected by an N=N double bond), which changes structure upon light exposure, and introduced this structure into existing metathesis catalysts to obtain a catalyst whose structure changes with light. The developed catalyst showed dramatic differences in reactivity depending on light exposure (60 to 300 times), which is much greater than the reactivity difference (1.5 to 2.5 times) of existing catalysts using the same strategy. They also demonstrated that changing the light irradiation conditions during the reaction could repeatedly switch the reactivity on and off.
Professor Hong said, "This research achievement is significant as it is the first catalyst case that can stop a reaction with light," adding, "Using the developed on-off switching catalyst, it is expected to apply light-based patterning technologies such as photolithography, which draws extremely fine and complex electronic circuits on semiconductor substrates to create integrated circuits."
The research results were published online on the 1st of this month in 'ACS Catalysis,' published by the American Chemical Society.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















