UNSIT Professor Park Cheolmin Team
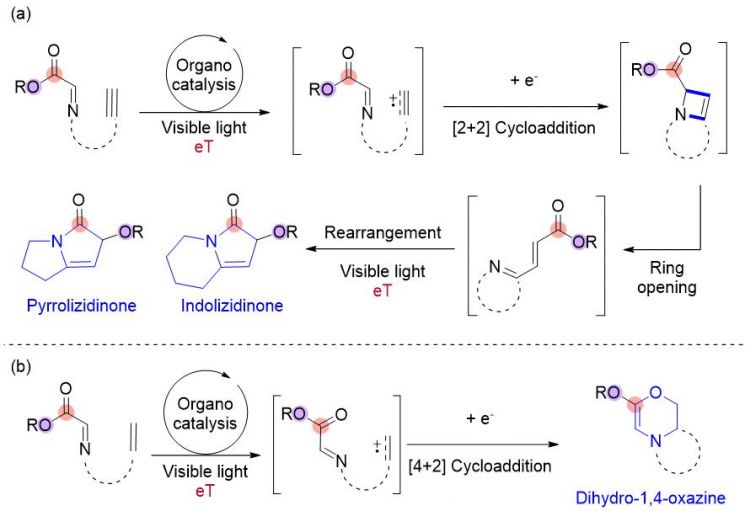 Schematic diagram of the synthesis method for polycyclic nitrogen compounds using organic photocatalysts and low-energy light. Image courtesy of UNIST
Schematic diagram of the synthesis method for polycyclic nitrogen compounds using organic photocatalysts and low-energy light. Image courtesy of UNIST
[Asia Economy Reporter Kim Bong-su] A new catalytic technology has been developed to synthesize nitrogen heterocycles, common components in major drugs, in an inexpensive and eco-friendly manner.
Ulsan National Institute of Science and Technology (UNIST) announced on the 11th that Professor Park Cheol-min's research team in the Department of Chemistry developed a technology to synthesize nitrogen heterocycles using inexpensive organic photocatalysts and low-energy visible light. This overcomes the drawbacks of existing metal catalyst-based technologies, such as toxicity risks from residual metal catalysts and high costs, raising expectations for eco-friendly commercialization.
The research team succeeded in synthesizing polycyclic nitrogen compounds such as pyrrolizidine derivatives and oxazine derivatives using this technology. Nitrogen heterocycles are compounds in which nitrogen atoms are incorporated into ring-shaped hydrocarbon structures. Due to the bio-molecular affinity of nitrogen components, over 75% of FDA-registered drugs consist of nitrogen heterocycles.
The method developed by the research team uses organic photocatalysts activated by low-energy visible blue light instead of metal catalysts, unlike conventional methods. Since the reaction is redox-neutral, no separate oxidizing or reducing agents are required, and it is an environmentally friendly method that can be performed at room temperature. Using this synthesis method, they reacted alkynes with imines to obtain pyrrolizidine structures composed of two fused five-membered nitrogen rings and indolizidine structures composed of fused five- and six-membered nitrogen rings. Pyrrolizidine and indolizidine serve as basic structures for antiarrhythmic agents and anticancer drug candidates, respectively. Additionally, using alkenes instead of alkynes can yield oxazine derivatives.
The research team elucidated the synthesis mechanism through additional experiments and Density Functional Theory (DFT). They discovered that a four-membered ring intermediate called azeitne is formed during the synthesis reaction. The principle is that alkynes oxidized by the photocatalyst react with imines to synthesize azeitne.
Oh Hyun-ji, a doctoral researcher in the Department of Chemistry at UNIST, explained, “The four-membered ring of azeitne has a strong tendency to open due to ring strain, leading to ring-opening reactions, which then undergo ring-closure reactions to ultimately synthesize pyrrolizidine derivatives.”
Professor Park said, “This research developed an inexpensive and environmentally friendly technology for synthesizing nitrogen polycyclic structures using organic catalysts instead of metal catalysts. It is expected not only to allow the attachment of various functional groups to nitrogen ring structures but also to be applicable to the synthesis of various other ring structures beyond nitrogen rings.”
The research results were published on the 26th of last month in the international journal 'ACS Catalysis.'
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![From Bar Hostess to Organ Seller to High Society... The Grotesque Con of a "Human Counterfeit" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















