-KAIST, Elucidation of the Thermochemical CO2 Reduction Mechanism on Perovskite
-Professor Jaewoo Lee's Research Team Successfully Develops and Optimizes CO2 Reduction Catalysts
-Expected to Contribute to Early Realization of Carbon Capture Technology in the Carbon Neutral Era
[Asia Economy Reporter Kim Bong-su] A technology has been developed to efficiently capture and reduce carbon dioxide using perovskite, which is gaining attention as a next-generation solar material.
The Korea Advanced Institute of Science and Technology (KAIST) announced on the 13th that a research team led by Professor Jae-woo Lee from the Department of Biological and Chemical Engineering succeeded in elucidating the mechanism of the thermochemical reduction reaction of carbon dioxide occurring on perovskite and diversifying factors to optimize the reaction.
Perovskite is a cubic structured metal oxide with the molecular formula ABO3 (A = lanthanide, B = transition metal) and is known as a material applied in next-generation solar cells.
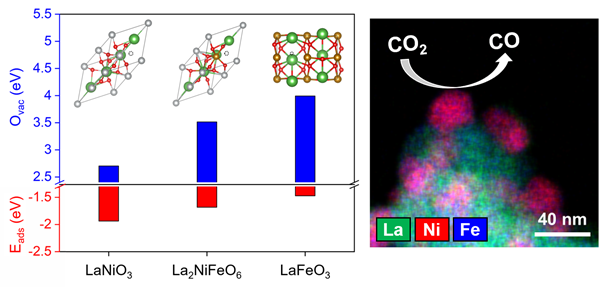
To predict the performance of the carbon dioxide reduction reaction, the research team confirmed that, in addition to the oxygen vacancy formation energy calculation, which has been mainly used so far, analyzing hydrogen adsorption energy, ionic conductivity, and the adsorption state of carbon dioxide can further improve the accuracy of performance prediction. Through the diversified factors achieved by the research team, it is expected that the performance of multidisciplinary carbon dioxide conversion and reduction catalysts to be developed for realizing carbon neutrality can be predicted more accurately.
The research results were published online on the 17th of last month in the international journal ACS Catalysis and were also selected as a cover paper.
Perovskite is attracting attention as a material that can maintain its structure stably even at high temperatures and through continuous oxidation-reduction cycles, making it applicable to carbon dioxide reduction reactions and water splitting reactions. However, previously, only the 'oxygen vacancy formation energy' was used as a factor to predict the performance of carbon dioxide reduction reactions on perovskites of various compositions, which had the drawback of somewhat lower accuracy.
The research team synthesized lanthanum-nickel-iron oxide (La2NiFeO6 molecular formula) double perovskite and conducted comparative analyses with lanthanum-nickel oxide (LaNiO3) and lanthanum-iron oxide (LaFeO3). They confirmed that the nickel (Ni) sites in the perovskite enhance particle reduction not only by forming oxygen vacancies but also by improving hydrogen adsorption and ionic conductivity, while the iron (Fe) sites prevent strong adsorption of carbon dioxide, promoting the dissociation reaction of carbon dioxide. Consequently, in La2NiFeO6 double perovskite, the roles of each site manifest synergistically, showing significantly superior carbon dioxide conversion compared to each single perovskite, confirming that all these factors can be utilized to predict performance.
Professor Lee explained, "Perovskite can be mass-produced, and when produced with optimized compositions through screening processes, it will contribute to the early realization of carbon capture, utilization, and storage (CCUS) technology that converts and utilizes carbon dioxide."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.