Korea Research Institute of Chemical Technology Developed AIDS Treatment in 2008
Passed Clinical Phases 1-3 Last June, Approved for Sale by Chinese Health Authorities
Aiming to Enter the 16 Trillion Won Global Market Dominated by Gilead
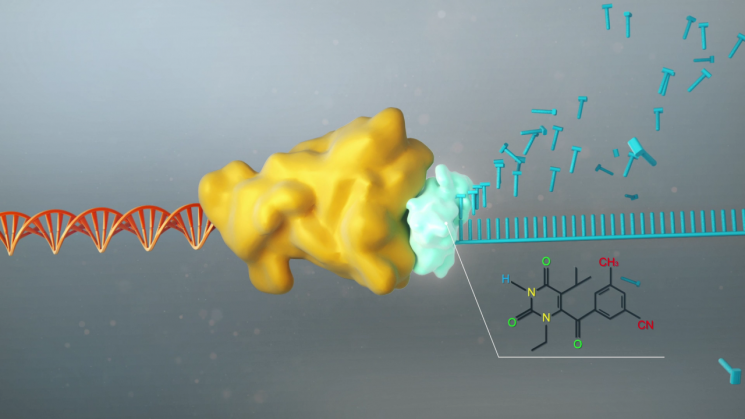 Schematic diagram of AIDS drug mimicking the function of reverse transcriptase enzyme inhibition. Image source=Korea Research Institute of Chemical Technology
Schematic diagram of AIDS drug mimicking the function of reverse transcriptase enzyme inhibition. Image source=Korea Research Institute of Chemical Technology
[Asia Economy Reporter Kim Bong-su] A treatment for acquired immunodeficiency syndrome (AIDS), developed domestically, has been recognized for its effectiveness in China and received approval for new drug sales. It is evaluated that this opens the possibility to target the global AIDS treatment market, which is dominated by global pharmaceutical companies and worth approximately 16 trillion KRW annually.
The Korea Research Institute of Chemical Technology announced on the 30th that the AIDS virus treatment developed by the research team of Dr. Son Jong-chan and Dr. Lee Il-young received sales approval from the China National Medical Products Administration (NMPA) last June.
The research team conducted continuous studies since 1995, discovered a candidate substance in 2008, and transferred the technology to Kainosmed in 2012. Subsequently, to commercialize the drug in China, where the number of AIDS patients is rapidly increasing, Kainosmed transferred the Chinese distribution rights of the candidate substance to the Chinese pharmaceutical company Jiangsu Aidi Pharmaceutical in 2014. After completing clinical phases 1 to 3 in China, the drug received sales approval from the NMPA in June this year.
The treatment discovered by the research team is a non-nucleoside reverse transcriptase inhibitor (NNRTI). Reverse transcriptase is a specific enzyme possessed by the AIDS virus (HIV) that converts the virus's RNA genetic information into DNA genetic information, playing a key role in HIV proliferation. By inhibiting the activity of reverse transcriptase, the proliferation of the AIDS virus can be blocked. Clinical trials in China showed that this treatment has fewer neuropsychiatric side effects and minimizes genetic toxicity. It also demonstrates excellent antiviral effects, can be administered orally once a day, and can be used in combination with other drugs.
So far, more than 30 million people worldwide have died from the AIDS virus. According to the WHO, there were 1.7 million new HIV infections* and AIDS patients worldwide in 2018. Although new infections are on a declining trend, some countries, including South Korea, are experiencing an increase in new cases. In particular, China recorded a cumulative total of 1.25 million HIV infections and AIDS patients in 2018, with approximately 80,000 new infections annually. This is estimated to be the highest increase rate for a single country, and the Chinese AIDS virus treatment market is estimated to be worth over 1 trillion KRW.
Previously, patients infected with the AIDS virus had a high probability of death due to immune system weakening, but after the development of treatments, they can survive and live daily lives by managing it like a chronic disease. Therefore, the importance of treatments has increased. Until now, Gilead has held a high market share globally for AIDS virus treatments, and the global market for AIDS virus treatments is currently estimated at 16 trillion KRW. Kainosmed, which holds the global distribution rights, plans to expand into other countries as well.
Dr. Son, who was responsible for the research while working at the Korea Research Institute of Chemical Technology until 2013, said, "This is a significant case as the first new drug candidate developed at the institute to be approved as a new drug. I hope it will greatly help AIDS virus-infected patients in China."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.