Flu Treatment with a Single Intravenous Injection
[Asia Economy Reporter Kim Ji-hee] JW Pharmaceutical announced on the 17th that it has launched the intravenous influenza treatment 'Fluenpera Joo' and started marketing activities.
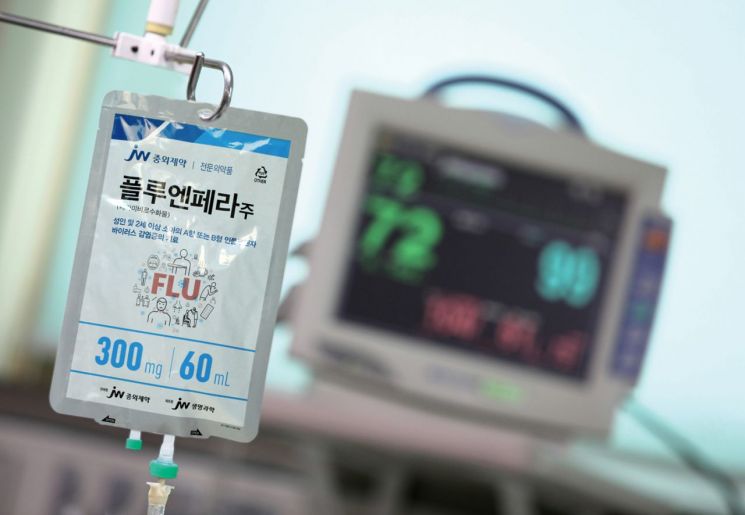
Fluenpera Joo is a peramivir hydrate formulation that treats influenza with a single intravenous injection, unlike oseltamivir formulations that require oral administration for 5 days. It is indicated for influenza virus infections of type A or B in adults and children aged 2 years and older.
Fluenpera Joo is the first domestically premixed intravenous influenza treatment in the form of an infusion bag, combining peramivir hydrate and saline. Previously released vial-type influenza injections required mixing the drug with a basic infusion solution. Additionally, this product is the first influenza treatment sold domestically to apply easy-cut technology, allowing only the bottom part of the aluminum packaging to be opened to connect the infusion set.
A JW Pharmaceutical official stated, "Reflecting the needs of medical sites, we developed an influenza infusion treatment that can alleviate inconveniences during the drug mixing process," and added, "We will expand market share through marketing that emphasizes the product's features, which enhance preparation convenience for patient treatment."
Production is handled by JW Life Science. JW Life Science has introduced many of the first premixed infusion solutions in Korea, applying proprietary technology, including Levitaram Injection (levetiracetam) in 2016, Acetapen Injection (acetaminophen) in 2019, and Jsedex Injection (dexmedetomidine hydrochloride) in 2020.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)










