Professor Joo Sang-hoon’s Chemistry Team at Ulsan National Institute of Science and Technology Develops Carbon-Based Catalyst for Electrochemical Hydrogen Peroxide Production
[Asia Economy Reporter Kim Bong-su] A high-performance catalyst that increases the commercial viability of an eco-friendly electrochemical hydrogen peroxide production method has been developed. This catalyst exhibits the highest activity and reaction efficiency among reported carbon-based catalysts. In particular, the core design elements of the catalyst have been identified, which is expected to guide future catalyst development.
The research team led by Professor Joo Sang-hoon of the Department of Chemistry at Ulsan National Institute of Science and Technology (UNIST) announced on the 9th that they have developed a carbon-based catalyst used for eco-friendly electrochemical hydrogen peroxide production. Unlike conventional hydrogen peroxide production methods that involve complex processes, the electrochemical method is simple and does not emit pollutants. Developing an inexpensive and high-performance catalyst was key to the commercialization of this method.
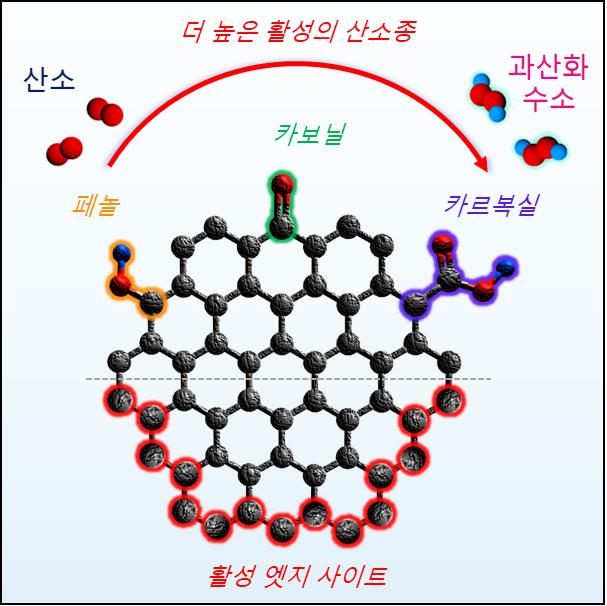
Among these, carbon-based catalysts have attracted attention due to their very low cost. However, during the ‘catalyst activation’ process to enhance the performance of carbon-based catalysts, indiscriminate structural changes occur, making it difficult to experimentally identify the core factors for catalyst performance and limiting catalyst development.
The research team used a synthesis method that can precisely control the structure of carbon catalysts to identify the key catalyst design elements that improve the performance and efficiency of carbon-based catalysts, and utilized this to develop a high-performance nanopororous carbon catalyst. Junseong Lim, a doctoral researcher in the Department of Chemistry and the first author, explained, “We identified carboxyl functional groups and edge carbon atoms as the core catalytic active sites, and based on this, we were able to develop a high-performance catalyst with a maximized number of active sites.”
The performance of the catalyst possessing a large number of active sites was the highest among carbon-based catalysts reported to date. It stably produced hydrogen peroxide for 168 hours without performance degradation and showed nearly 100% efficiency. Electrical energy was not wasted producing water (H2O) as a byproduct, and almost 100% hydrogen peroxide was generated, resulting in high efficiency.
Hydrogen peroxide, widely used from wound disinfection to semiconductor cleaning, currently relies on the anthraquinone process. This process requires expensive palladium catalysts and releases organic pollutants. In contrast, the electrochemical production method produces no reaction byproducts other than water (H2O), and when combined with electricity generated from renewable energy, production costs can also be reduced.
Professor Joo stated, “This study, which presents the core design secrets of carbon-based catalysts, is expected to serve as an important guideline for future catalyst development,” adding, “It brings us one step closer to electrochemical hydrogen peroxide production that can replace the anthraquinone process, which generates large amounts of organic pollutants.”
This research was published online on the 30th of last month in Chem, a sister journal of Cell.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.