Korea Institute of Machinery and Materials Proposes High-Efficiency Production Method Using Renewable Energy at Room Temperature and Pressure
[Asia Economy Reporter Kim Bong-su] Domestic researchers have developed an innovative ammonia production process that emits no carbon at all. Ammonia is considered a future-oriented clean energy source because it does not emit carbon dioxide when combusted, allowing it to be used directly as fuel, and it contains a large amount of hydrogen, making it useful as a 'hydrogen carrier.'
The Korea Institute of Machinery and Materials announced on the 19th that the research team led by Principal Researcher Lee Dae-hoon of the Plasma Research Laboratory succeeded in developing an innovative carbon-free ammonia production process that produces ammonia at room temperature and atmospheric pressure using renewable energy.
This research result was published on the 5th in the energy science journal ACS Energy Letters.
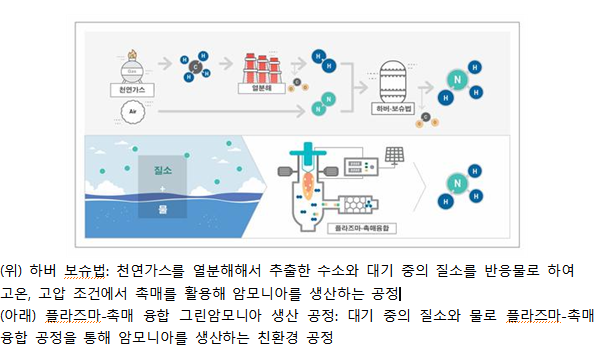
The conventional ammonia synthesis method is the 'Haber-Bosch process,' developed by Fritz Haber and Carl Bosch in 1913. A 2020 report by the Royal Society of the United Kingdom states that 1.8% of the world's energy is used to produce ammonia via the Haber-Bosch process, and the carbon dioxide emissions from this process account for 1.8% of global emissions. The Haber-Bosch process requires high-pressure conditions above 200 atmospheres and high temperatures above 400°C to synthesize ammonia. Additionally, fossil fuels such as natural gas are used to obtain hydrogen, resulting in high energy consumption and significant carbon dioxide emissions.
The research team developed a process that supplies water to nitrogen plasma, decomposes it to produce hydrogen and nitrogen oxides, and then supplies these to a catalyst to produce ammonia. More than 99% of the nitrogen oxides produced by this plasma reaction are in the form of nitric oxide, which can be easily synthesized into ammonia. The synthesized nitric oxide reacts with the hydrogen produced simultaneously to synthesize ammonia with a high selectivity of over 95%, and the heat required for this reaction is supplied by the heat generated during the plasma decomposition process.
This process does not use any fossil fuels and produces 'green ammonia' with zero carbon dioxide emissions. Compared to various electrochemical ammonia production methods proposed as alternatives to the Haber-Bosch process, it achieves 300 to 400 times higher yield.
Unlike the conventional high-temperature, high-pressure process, ammonia production is possible at room temperature and atmospheric pressure using only water and nitrogen, and the modular system allows relatively easy scale-up of the process. Additionally, the byproduct nitrate (Nitrate) aqueous solution generated alongside ammonia production can be usefully applied as agricultural nutrient solutions or oxidizers.
The research team plans to improve ammonia production costs and efficiency through future mass production and product development technologies and seek to enter the ammonia plant sector, especially small to medium-sized plants capable of operating at atmospheric pressure and room temperature, in collaboration with domestic and international engineering companies.
Principal Researcher Lee Dae-hoon said, “By using electricity based on renewable energy, we have theoretically developed an environmentally friendly ammonia production process with zero carbon emissions that is applicable to actual facilities,” adding, “We will devote ourselves to follow-up research and development to help achieve a revolutionary reduction in global carbon emissions.”.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.


![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)














