Korea Advanced Institute of Science and Technology Develops 'Virtual Drug Screening' Technology to Identify COVID-19 Effective Substances Among Existing Drugs
Clinical Trials Planned to Determine Optimal Dosage After Toxicity Found in Animal Testing
[Asia Economy Reporter Kim Bong-su] Domestic researchers have devised a new method to rapidly identify substances effective against the COVID-19 virus from thousands of existing drugs. Notably, although toxicity was detected, they discovered a COVID-19 treatment candidate substance that is 200 times more effective than the U.S. drug remdesivir (Veklury), drawing attention to future developments.
The Korea Advanced Institute of Science and Technology (KAIST) announced on the 8th that a joint research team led by Distinguished Professor Lee Sang-yeop of the Department of Bio and Chemical Engineering at KAIST and Dr. Kim Seung-taek of the Korea Pasteur Institute succeeded in developing a COVID-19 treatment using virtual drug screening technology.
Remdesivir has been officially approved by the U.S. Food and Drug Administration (FDA) for COVID-19 treatment, but it has been evaluated as falling short of expectations because it does not reduce mortality and only shortens recovery time by about five days. Since it is administered intravenously and requires hospitalization for several days, it is not suitable for pandemic situations, highlighting the urgent need for oral treatments.
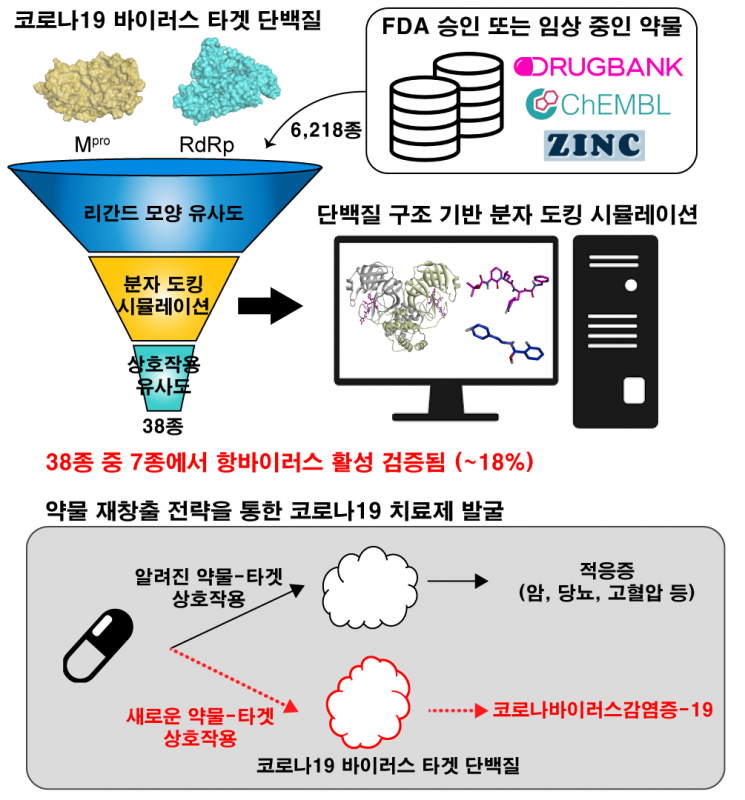
Based on these points, the research team conducted COVID-19 treatment development using a drug repurposing strategy with virtual drug screening technology. Dr. Jang Woo-dae of the KAIST research team first collected FDA-approved drugs or those in clinical trials from databases to build a virtual library of 6,218 drugs. Because experimentally verifying all these drugs would require significant time and cost, they introduced a computer-based virtual screening technology to quickly select drugs with potential as antiviral agents.
Existing docking simulation-based virtual screening technologies had very low hit rates due to high false positive rates. To address this, the research team introduced structural similarity analysis and interaction similarity analysis modules before and after docking, successfully improving the accuracy of virtual screening. The virtual screening technology developed in this study is characterized by its ability to rapidly and accurately screen various candidate drugs using protein-drug complex structural information.
The team also developed an algorithm that automatically generates the active form structures of nucleotide analogue-based prodrugs, which are mainly used as antiviral agents. Prodrugs themselves have no efficacy and must be metabolized into active forms in the body to exhibit therapeutic effects. Therefore, it is important to perform docking simulations after converting prodrugs into their active forms. The team succeeded in automatically generating active form structures of several nucleotide analogue-based prodrugs, including remdesivir, enhancing the accuracy of docking simulations.
Through this, the team selected 15 and 23 candidate compounds, respectively, that can inhibit the 3CL hydrolase (Mpro) and RNA-dependent RNA polymerase (RdRp), proteins essential for the replication and proliferation of SARS-CoV-2.
Subsequently, the antiviral efficacy of 38 drugs selected by virtual screening was verified using a cell image-based antiviral activity analysis platform in a biosafety level 3 (BSL-3) laboratory at the Korea Pasteur Institute.
First, in vitro experiments using Vero cells (monkey kidney cells) infected with SARS-CoV-2 confirmed antiviral activity in 7 of the 38 drugs. Additional verification experiments were conducted on these 7 drugs using human lung cells (Calu-3 cells).
The research team particularly confirmed antiviral activity in three drugs: omipalisib, currently in clinical trials for cancer and idiopathic pulmonary fibrosis; tipifarnib, in clinical trials for cancer and progeria; and emodin, a plant extract undergoing clinical trials as an anticancer agent.
Notably, omipalisib showed antiviral activity approximately 200 times higher than remdesivir, the current standard COVID-19 treatment, while tipifarnib demonstrated antiviral activity comparable to remdesivir.
However, through the Ministry of Science and ICT’s preclinical support project for COVID-19 treatments, toxicity was observed in animal experiments evaluating one of the candidate drugs. Accordingly, the research team plans to conduct additional preclinical trials to find the optimal drug concentration that minimizes toxicity while achieving therapeutic efficacy. Preclinical trials are also planned for the other candidate drugs.
Distinguished Professor Lee Sang-yeop of KAIST stated, "This research is significant in establishing a foundational technology for rapid response to emerging novel viruses," adding, "We aim to develop technologies applicable to similar coronaviruses or newly emerging viruses in the future."
The research results were published online on the 7th in the international journal Proceedings of the National Academy of Sciences (PNAS).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.