Slowing Cognitive Decline Speed
Annual Treatment Cost Around 60 Million KRW
[Asia Economy Reporter Cho Hyun-ui] The U.S. Food and Drug Administration (FDA) approved Aducanumab (brand name Aduhelm), a new Alzheimer's drug jointly developed by U.S. Biogen and Japan's Eisai, on the 7th (local time). This is the first Alzheimer's drug to receive FDA approval in 18 years since 2003.
The FDA stated, "Aducanumab removes beta-amyloid, a toxic protein that accumulates in the brains of Alzheimer's patients," and added, "We have decided to approve it because it can reasonably predict significant effects for patients."
Aducanumab is the first drug to slow the decline of cognitive functions such as memory and language abilities in Alzheimer's patients. While it cannot reverse mental deterioration, it has the effect of delaying the progression of the disease.
Foreign media noted, "The drug approved in 2003 managed symptoms such as anxiety and insomnia," and evaluated, "This is the first time a new drug developed to address the fundamental cause of the disease has been approved."
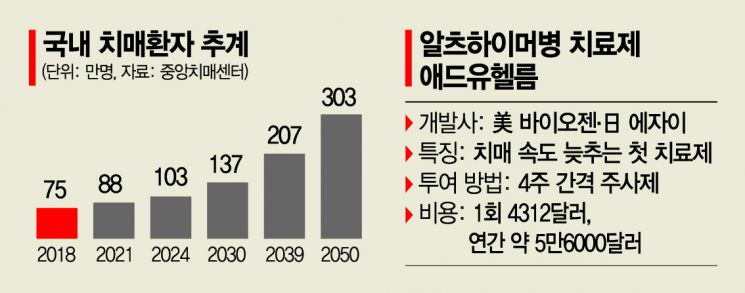
The cost per dose is set at $4,312 (approximately 4.8 million KRW). Considering it must be administered via injection once every four weeks, the annual cost amounts to about $56,000 (approximately 62.3 million KRW).
However, the FDA imposed a requirement for Biogen to conduct follow-up studies to confirm the efficacy of Aducanumab. The 'conditional approval' essentially reflects ongoing controversy over its effectiveness.
Biogen claims that Aducanumab improves symptoms by breaking down amyloid plaque deposits that form clusters in the brains of Alzheimer's patients.
The New York Times reported, "Some doctors involved in Aducanumab's clinical trials express doubts about its efficacy," and added, "Even if some patients can slow Alzheimer's progression, the benefits gained from the drug may not outweigh risks such as bleeding."
Opposition also came from the FDA's own scientific advisory committee. In November last year, the advisory committee questioned the effectiveness of the new drug submitted by Biogen and ultimately opposed its approval.
In March last year, Biogen halted clinical trials after an interim analysis of Phase 3 showed no improvement in patients' memory and cognitive abilities. However, after reanalyzing clinical trial data from patients who received a high dose, Biogen reported that it delayed the decline in memory, cognitive ability, and daily functioning by up to 22%, which the FDA reviewed.
The high price, far exceeding experts' expectations, has also sparked controversy. Experts initially estimated the annual cost of Aducanumab to be between $10,000 and $25,000 (approximately 11.13 million to 27.81 million KRW).
Michael Bonatos, CEO of Biogen, stated, "Considering the fact that there has been no innovation for 20 years, the price is reasonable," and promised, "We will not raise the price for the next four years."
Alzheimer's is an incurable disease that gradually attacks areas of the brain necessary for memory, reasoning, communication, and basic daily tasks. According to the Central Dementia Center, the number of dementia patients aged 65 and older, which was 750,000 in 2018, is estimated to exceed 1 million by 2024, 2 million by 2039, and 3 million by 2050.
In the United States, about 6 million people suffer from it, and globally about 30 million. The World Health Organization (WHO) projects that the number of dementia patients worldwide will reach 150 million by 2050.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.