Korea Research Foundation "Professor Park Jaehyung's Research Team Demonstrates Reprogramming of Inflammatory Macrophages into Anti-Inflammatory Type"
[Asia Economy Reporter Kim Bong-su] A treatment method that targets inflammatory joint areas in rheumatoid arthritis to eliminate the cause has been developed by domestic researchers.
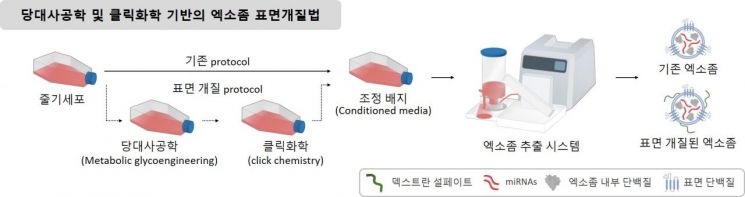
The National Research Foundation of Korea announced on the 6th that Professor Jae-Hyung Park's research team at Sungkyunkwan University developed targeted stem cell exosomes that selectively accumulate in inflammatory joint areas while circulating in the body and reprogram inflammatory macrophages into an anti-inflammatory type.
Inflammatory macrophages, also called M1 macrophages, are macrophages that cause inflammation and are a major cause of the worsening of rheumatoid arthritis. Exosomes are small vesicles secreted outside cells that contain large amounts of biomolecules such as DNA, proteins, and microRNA, and are involved in various physiological phenomena in our body. In particular, stem cell exosomes are known to convert inflammatory macrophages into anti-inflammatory ones, but they are rapidly degraded and disappear in the body and mainly accumulate in the liver rather than the inflammatory site, limiting their effectiveness.
The research team attempted surface modification so that stem cell exosomes could target inflammatory joint areas. They introduced dextran sulfate (a dextran-based polysaccharide with sulfate groups) onto the surface of stem cell exosomes, which can bind to the SR-A receptor abundantly expressed on the surface of inflammatory macrophages present in inflammatory sites.
The research team administered the stem cell exosomes created in this way intravenously to mice and confirmed through optical imaging devices that the exosomes relatively concentrated and accumulated in the inflammatory areas. In particular, mice with surface-modified exosomes accumulated in the inflammatory sites showed significantly lower arthritis scores compared to the control group. Even at one-tenth the dose compared to conventional exosomes, similar arthritis scores were observed.
The research team revealed that the anti-inflammatory effect of these exosomes was due to specific microRNAs (let-7b-5p and miR-24-3p). They also confirmed that glycoengineering for targeting (a technology that can introduce chemical reactors onto cell surface glycoproteins) and bioorthogonal copper-free click chemistry-based cell surface modification did not affect the biomolecules inside the exosomes. Bioorthogonal copper-free click chemistry refers to a phenomenon where azide and alkyne groups react specifically without a copper catalyst.
The results of this study were published on the 2nd in the international journal Science Advances.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















