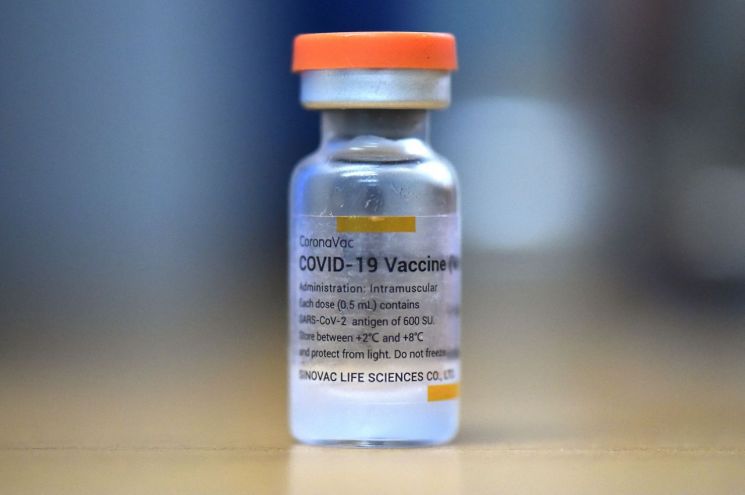
[Asia Economy Reporter Seongpil Cho] Chinese authorities have approved the emergency use of the Sinovac (Kexing·科興) COVID-19 vaccine for individuals aged 3 to 17, according to In Weidong (尹衛東), chairman of the company.
Chairman In appeared on a state-run CCTV program on the evening of the 4th and stated, "Sinovac has completed Phase 1 and Phase 2 clinical trials for the 3 to 17 age group, and the safety and efficacy of the vaccine have been proven in this group just as in adults." He added, "Although the emergency use age range for the Sinovac COVID-19 vaccine has been expanded from '18 years and older' to '3 years and older,' further review is needed to determine when it can be extended to younger age groups."
Currently, COVID-19 vaccinations are being administered nationwide in China to individuals aged 18 to 59. In some regions such as Beijing and Shanghai, elderly people aged 60 and above are also receiving the vaccine. Sinovac became the second Chinese company, after Sinopharm (China National Pharmaceutical Group), to receive emergency use approval for its COVID-19 vaccine from the World Health Organization (WHO) on the 1st.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















