Dr. Jeong Beomgyun and Research Team at KBSI Dramatically Enhance Performance of Existing Iron-Nitrogen-Carbon Catalysts
Taking a Step Closer to Industrial Applications in Drones, Exploration Robots, Kickboards, and Carts
[Asia Economy Reporter Kim Bong-su] A catalyst that dramatically improves the performance of the 'alkaline hydrazine liquid fuel cell' has been developed. This brings the industrial use of liquid fuel cells in drones, exploration robots, kickboards, carts, and more one step closer.
The Korea Basic Science Institute (KBSI) announced on the 26th that a joint research team led by Dr. Jeong Beom-gyun of the Materials Analysis Research Division succeeded in developing a catalyst that can raise the performance of alkaline hydrazine liquid fuel cells to the world's highest level.
Hydrogen fuel cells, which are gaining attention as eco-friendly power generation devices, have the best output performance, but special technology and equipment are required to store and transport hydrogen at high pressure without leakage. Liquid fuel cells are next-generation energy sources that can be used in transportation power devices without these technical issues.
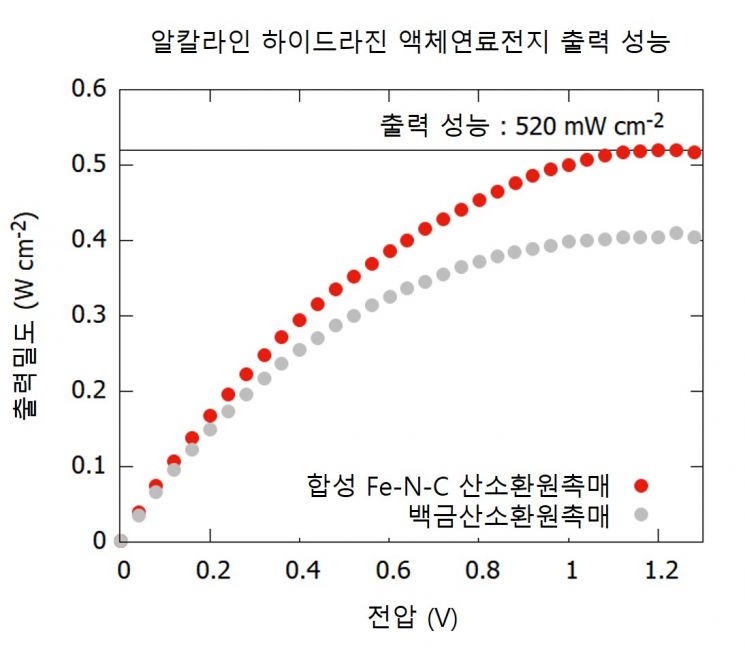
Among them, the 'hydrazine liquid fuel cell' has output performance comparable to hydrogen fuel cells and has the advantage as a future transportation power source because it can utilize existing storage and transportation infrastructure for liquid fuels. Fuel cells are classified into acidic fuel cells and alkaline fuel cells depending on the acidity of the electrolyte. The alkaline fuel cells studied by the research team this time are characterized by achieving high performance and stability without using expensive platinum catalysts.
In alkaline liquid fuel cells, iron-nitrogen-carbon catalysts are attracting attention as catalysts that can replace platinum, but they have the disadvantage of having a lower density of active sites (points where catalytic reactions occur) on the catalyst surface compared to platinum. Therefore, a relatively large amount of catalyst must be used to achieve the desired output performance. However, when a large amount of catalyst is used, the thickness of the catalyst layer applied to the electrode increases, which hinders material transport and prevents the fuel cell output from increasing proportionally to the amount of catalyst used.
The catalyst developed by the joint research team is an electrode catalyst whose structure and surface were modified through steam activation during the synthesis of the iron-nitrogen-carbon catalyst. The team used electrospinning to heat-treat the iron-nitrogen-carbon catalyst with high-temperature steam, forming a hierarchical pore structure on the catalyst surface suitable for fuel cell operating environments, allowing oxygen gas to easily access the active sites. They also confirmed that iron oxide (Fe₃O₄) nanoparticles generated during the heat treatment process reduce interfacial resistance between the electrode and electrolyte, maintaining output performance even at high currents.
Dr. Jeong Beom-gyun of KBSI stated, "It is meaningful that we identified that not only the pore structure but also the surface properties of the catalyst affect the performance of the fuel cell," adding, "We are conducting follow-up research to further refine the modified surface properties from this study to enhance output performance."
The results of this research were recently published online in the Journal of Energy Chemistry, a world-renowned academic journal in the field of chemistry.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.