Professor Jeon Gi-jun's Team at Inha University, Korea Research Foundation
[Asia Economy Reporter Kim Bong-su] Amid active research to obtain hydrogen environmentally from water instead of fossil fuels, a method to significantly reduce the cost of platinum catalysts used in the water electrolysis process has been developed by a domestic research team.
The National Research Foundation of Korea announced on the 3rd that Professor Jeon Ki-jun's research team from the Department of Environmental Engineering at Inha University proposed a new low-cost catalyst comparable to the expensive platinum catalyst essential for the water electrolysis process that splits water to obtain hydrogen.
As interest in green hydrogen energy as a next-generation energy source grows, research on water electrolysis technology to obtain hydrogen by splitting water is active. The excellence of platinum as a catalyst aiding water electrolysis is well known, but the key to commercialization was whether the high platinum content could be sufficiently reduced to an economically viable level.
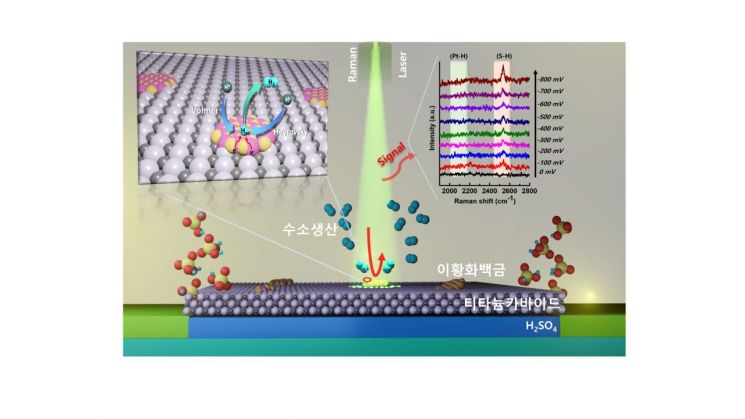
The research team lowered the platinum content to about 1/5000 of the existing cathode material (10% platinum) by using a platinum-aniline complex, where aniline is a compound containing a benzene ring, added to platinum. Based on this, they designed a platinum disulfide (PtS2) catalyst in the form of quantum dots with sulfur introduced, which improved both electrical efficiency and stability.
Instead of a separate process to attach the catalyst to the substrate, they also developed a method to directly grow platinum disulfide on the substrate using chemical vapor deposition. This was done to eliminate the need for a binder itself, preventing the decrease in stability caused by the reduced adhesion effect of the binder connecting the substrate and catalyst during the water electrolysis reaction. The titanium carbide substrate produced in this way showed about 12 times lower electrical resistance compared to the existing titanium dioxide substrate, and when applying the cathode electrode with platinum disulfide, it demonstrated hydrogen production efficiency almost equivalent to that of the cathode with the existing platinum catalyst. It also showed excellent stability, maintaining performance for over 60 hours even in extreme acidic environments.
This research result was published online on the 15th in the international journal in the energy and environment field, Applied Catalysis B: Environmental.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















