Announcement of Administrative Investigation Results on Suspected Minimum Residual Manufacturing at Uncertified Facilities
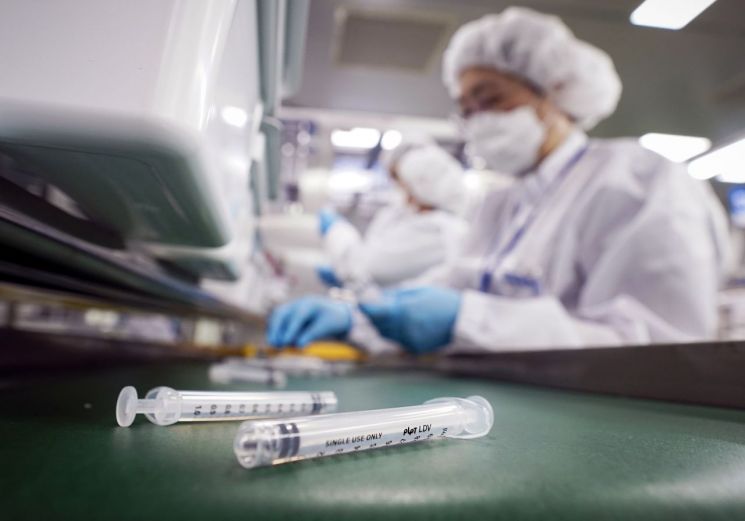 Employees of Punglim Pharmatech are producing low dead space (LDS) syringes.
Employees of Punglim Pharmatech are producing low dead space (LDS) syringes. [Image source=Yonhap News]
[Asia Economy Reporter Kim Heung-soon] The Ministry of Food and Drug Safety announced on the 29th that an administrative investigation into Poonglim Pharmatech, which was suspected of manufacturing low dead space (LDS) syringes for COVID-19 vaccination in an 'unauthorized' facility, confirmed no violations of the Medical Device Act.
Since the 23rd, the Ministry has conducted an administrative investigation to verify whether Poonglim Pharmatech produced LDS syringes and, if so, for what purpose. The investigation confirmed that the finished LDS syringes and components distributed domestically were manufactured at the main factory in Gunsan, Jeonbuk, which holds GMP certification for medical device manufacturing and quality control standards.
Earlier, suspicions arose that Poonglim Pharmatech manufactured LDS syringes at a new factory located in the Saemangeum Industrial Complex in Gunsan, Jeonbuk. Since the new factory has not yet received GMP certification, LDS syringes produced there should not be distributed domestically. The Ministry's investigation found that while components were produced at the new factory, they were partially used for production facility validation and prototype manufacturing for GMP certification, with the remainder being stored.
According to the Ministry, manufacturing for production facility validation, prototype production, and export products before GMP certification does not violate the Medical Device Act.
Although no violations of the Medical Device Act were found during the administrative investigation, the Ministry took measures to ensure that components manufactured and stored at the new factory are not used in products distributed domestically before GMP certification. Additionally, when these components are used in export products, the Ministry provided administrative guidance to ensure proper process management and thorough testing of components and finished products.
Poonglim Pharmatech is a manufacturer of LDS syringes, also known as 'K syringes.' LDS syringes are special syringes designed to minimize vaccine waste by having almost no space between the piston and the needle.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















