 Diagram illustrating the suspected manipulation of particle size data for pharmaceutical ingredients by Daewoong Pharmaceutical. Provided by the Korean Intellectual Property Office.
Diagram illustrating the suspected manipulation of particle size data for pharmaceutical ingredients by Daewoong Pharmaceutical. Provided by the Korean Intellectual Property Office.
[Asia Economy (Daejeon) Reporter Jeong Il-woong] Daewoong Pharmaceutical Co., Ltd. is now facing prosecution investigation over allegations of manipulating patent specifications.
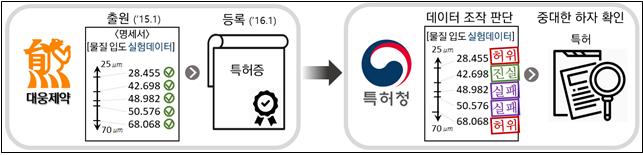
On the 29th, the Korean Intellectual Property Office (KIPO) announced that it judged Daewoong Pharmaceutical submitted manipulated key experimental data during the process of applying for and registering patents for the peptic ulcer drugs Albis and Albis D. Accordingly, the examiner requested the Patent Court to invalidate the patents ex officio and also requested the prosecution to investigate the case under the patent law’s “crime of false acts.”
The crime of false acts refers to applying for or registering a patent, extending the patent term registration, or receiving a decision based on false information. In such cases, the patent holder can be sentenced to imprisonment of up to three years or fined up to 30 million KRW according to Article 229 of the Patent Act.
In Daewoong Pharmaceutical’s case, it is KIPO’s judgment that in January 2016, the company manipulated most of the experimental data in the patent specification to obtain patent registration. Furthermore, during the patent invalidation trial, the company testified as if the manipulated data were “truthful,” and in January 2017, received a decision that the patent registration it obtained was not invalid, which constitutes the crime of false acts.
Previously, Daewoong Pharmaceutical was also subject to fines and corrective measures by the Fair Trade Commission (FTC) for the same issue. The FTC also viewed that Daewoong Pharmaceutical obtained the patent by manipulating experimental data. Moreover, judging this act as unfair trade practice, the FTC decided last month to impose a fine of 2.3 billion KRW.
In this regard, KIPO plans to strengthen prior cooperation with the FTC to ensure consistency in handling patent-related cases.
In particular, KIPO intends to strictly deal with acts like Daewoong Pharmaceutical’s, which involve manipulating important experimental data to fraudulently obtain patents, considering them as cases that undermine the fairness of the patent system.
Kim Ji-soo, Director of the Patent Examination Planning Bureau at KIPO, said, “As the economic value of patents rises due to the expansion of intellectual property financing and the introduction of punitive damages, establishing a fair patent system is more important than ever.” He added, “KIPO will firmly respond to acts of fraudulently obtaining patents by falsifying documents.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
!["The Woman Who Threw Herself into the Water Clutching a Stolen Dior Bag"...A Grotesque Success Story That Shakes the Korean Psyche [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















