Conditional Approval by MFDS... Available for Use Next Month
Low Sensitivity and Difficult for Self-Collection
"Not for Diagnosis... Should Only Be Used as an Auxiliary Tool"
[Asia Economy Reporter Seo So-jeong] Starting next month, self-test kits that allow individuals to check for COVID-19 infection at home without medical personnel will be available for purchase at pharmacies and online.
On the 23rd, the Ministry of Food and Drug Safety conditionally approved two types of self-test kits from two manufacturers, SD Biosensor and Humasis. Conditionally approved products can be used temporarily (for 3 months) before the official products are released.
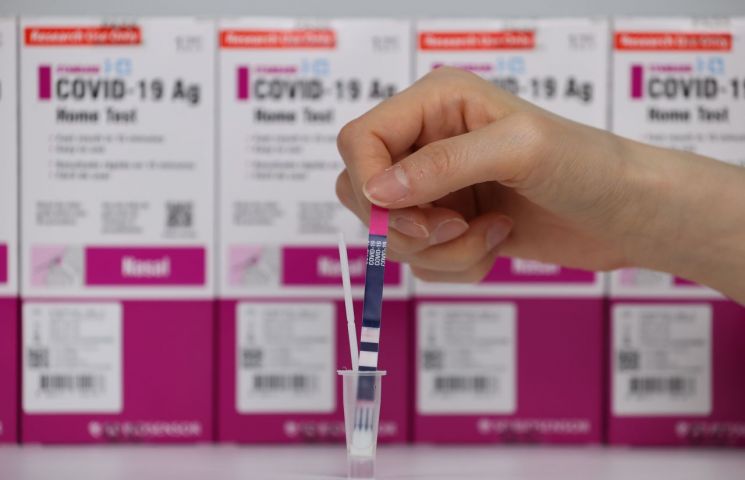
These products allow individuals to collect samples directly from their nasal cavity and visually check the results within 15 to 20 minutes. A Ministry of Food and Drug Safety official stated, "These two products have submitted clinical performance data for self-testing conducted abroad and have been approved for use in countries within Europe," adding, "It is expected that they will be available for purchase at pharmacies and online about 7 to 10 days after approval."
The Ministry explained that the two products should only be used as supplementary tools, not for confirming COVID-19 diagnosis.
Opinions in the medical community regarding the use of self-test kits are divided. On the day when the number of new COVID-19 cases reached 797, maintaining the 700s for three consecutive days amid a growing spread, there is hope that improved accessibility to testing will help identify 'asymptomatic transmitters.'
However, there are significant counterarguments. The biggest issue is accuracy. Most self-test kits use antigen testing methods to quickly provide results, but their sensitivity is lower than that of polymerase chain reaction (PCR) tests, resulting in significantly reduced accuracy.
Earlier this year, a research team led by Professor Kim Nam-joong of Seoul National University Hospital’s Department of Infectious Diseases reported that the accuracy of antigen test products was only 17.5% compared to PCR tests. False positives could lead to unnecessary isolation and work disruptions, while false negatives could result in virus spreaders, potentially causing a backlash against quarantine efforts.
There is also uncertainty about whether sample collection by the general public will be properly conducted. Professor Kim Ja-young of the Department of Laboratory Medicine at International St. Mary’s Hospital said, "Self-test kits require sample collection from the nasal cavity (near the entrance of the nostrils), but it is not as easy as expected for the general public to collect samples inside the nostrils."
Professor Kim emphasized, "It is very important to consider how the clinical target group (evaluation population) was selected when validating self-test kits. If the evaluation population consisted of symptomatic individuals, sensitivity would be high, but if asymptomatic individuals were included, sensitivity would inevitably be lower. Therefore, the sensitivity and specificity of the kit should be evaluated considering prevalence."
Currently, since the prevalence rate in Korea is not high, the kits are likely to be used to prevent asymptomatic transmission. It is pointed out that careful scrutiny is needed to verify whether data validation for this purpose has been properly conducted.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
!["The Woman Who Threw Herself into the Water Clutching a Stolen Dior Bag"...A Grotesque Success Story That Shakes the Korean Psyche [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















