[Asia Economy Honam Reporting Headquarters Reporter Lee Gwan-woo] The research team led by Professor Choi Chang-hyuk of the Department of New Materials Engineering at Gwangju Institute of Science and Technology (GIST) announced on the 19th that they have secured a fundamental technology for performance evaluation of non-precious metal catalysts for hydrogen fuel cells.
This is being evaluated as a step closer to the commercialization of hydrogen fuel cells.
Hydrogen fuel cells, which produce electricity by reacting hydrogen with oxygen in the air, require catalysts to facilitate these reactions.
Existing hydrogen fuel cells mainly used platinum, a precious metal, as a catalyst, but the scarcity and high cost of platinum have been major obstacles to commercialization.
Therefore, recent research has been actively conducted on inexpensive catalysts that can replace platinum.
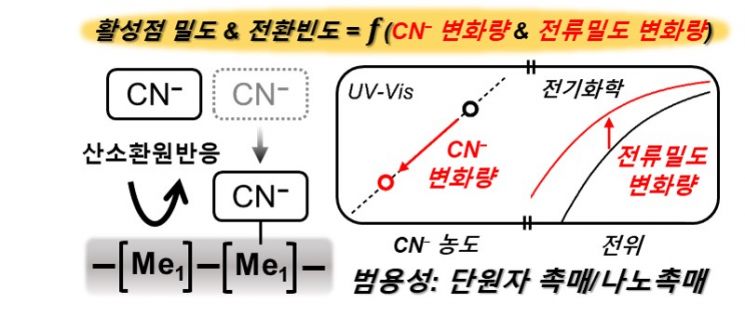
Among them, single-atom iron-based catalysts composed of iron, nitrogen, and carbon have attracted attention as efficient oxygen reduction reaction catalysts, but due to the lack of performance diagnostic technology for single-atom catalysts, it has been difficult to identify key performance determinants such as active site density and turnover frequency.
The oxygen reduction reaction refers to the reaction occurring at the anode of a hydrogen fuel cell, where oxygen reacts with electrons and protons to be reduced to water.
Active site density is the density of sites on the catalyst surface where reactants undergo catalytic action.
Turnover frequency refers to the number of reactant molecules reacting per active site of the catalyst per unit time.
The research team devised a technology to measure active site density and turnover frequency, key determinants of catalyst performance, based on the fact that cyanide ions (CN-) can irreversibly adsorb onto iron-based single-atom catalysts.
Using a specially designed reactor, cyanide ions were irreversibly adsorbed onto the iron-based single-atom catalyst. The amount of adsorbed cyanide ions was quantified by ultraviolet-visible (UV-Vis) spectroscopy, and simultaneously, the decrease in oxygen reduction reactivity of the catalyst with irreversibly adsorbed cyanide ions was measured.
Through this, they successfully derived the catalyst's active site density and turnover frequency.
Furthermore, by deriving performance determinants of single-atom catalysts based on other transition metals and precious metals, as well as widely used commercial platinum catalysts, they demonstrated the universality of the developed diagnostic technology.
Professor Choi Chang-hyuk said, “The greatest significance of this research achievement is that we have secured a fundamental technology for performance evaluation of iron-based single-atom catalysts, which are close to commercialization recently,” adding, “We hope that by utilizing this technology in the future to develop highly active oxygen reduction catalysts, we can contribute to the activation of the future hydrogen economy.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.