Conducted on 600 Patients in Severe High-Risk Group
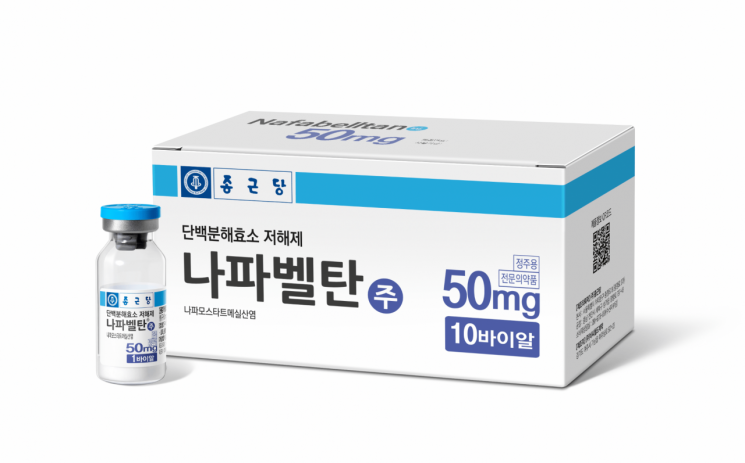
[Asia Economy Reporter Kim Ji-hee] Chong Kun Dang announced on the 16th that it has received approval from the Ministry of Food and Drug Safety for the Phase 3 clinical trial plan of 'Napabeltan (Nafamostat)', which is being developed as a treatment for COVID-19.
This Phase 3 clinical trial will be conducted domestically at more than 10 institutions, including Seoul National University Hospital, targeting about 600 severe high-risk patients. Chong Kun Dang also plans to conduct global clinical trials in Europe, Brazil, Russia, and India to rapidly recruit clinical patients.
Last year, Chong Kun Dang conducted a Phase 2 clinical trial of Napabeltan on 104 severe COVID-19 patients in Russia. Based on these results, it also applied for conditional approval from the Ministry of Food and Drug Safety. However, at that time, the Ministry stated that the Phase 2 results alone were insufficient to recognize the treatment effect for COVID-19 and expressed the opinion that additional clinical trials confirming the treatment effect were necessary.
A Chong Kun Dang official said, “In the absence of COVID-19 treatments for severe high-risk patients, we plan to prove the therapeutic effect of Napabeltan through a large-scale Phase 3 clinical trial,” adding, “If Napabeltan is supplied as a COVID-19 treatment by rapidly conducting clinical trials both domestically and internationally, it could contribute to reducing the mortality rate caused by COVID-19.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















