[Asia Economy Honam Reporting Headquarters Reporter Lee Gwan-woo] Domestic researchers have developed an electrode catalyst and succeeded in achieving top-level performance of formate liquid fuel cells produced from carbon dioxide.
The research team led by Professor Jae-young Lee of the Department of Earth and Environmental Engineering at Gwangju Institute of Science and Technology (GIST) announced on the 12th that they developed an oxidation electrode catalyst used in alkaline formate fuel cells.
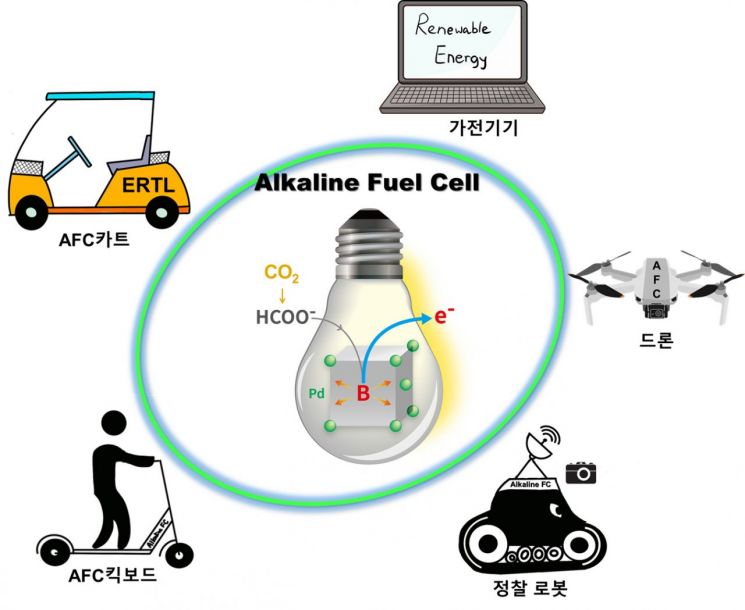
The team introduced boron into palladium metal in the formate fuel cell and utilized it as an electrochemical catalyst, improving the fuel cell's performance by enhancing the formate oxidation kinetics of the catalyst.
Palladium (Pd) has traditionally been used mainly for oxidation and reduction reactions of organic compounds, and in the electrochemical field, it shows excellent oxidation performance as an oxidation electrode catalyst alongside platinum (Pt).
In particular, Pd's oxidation performance in formate oxidation greatly surpasses that of Pt, and research on developing Pd-based electrode catalysts is underway.
Formate can be directly mass-produced through the electrochemical reduction process of carbon dioxide, enabling the realization of a carbon-neutral fuel cell system and allowing higher current and power density output compared to other fuels in fuel cell systems.
Liquid fuel cell systems can be used in electronic devices such as laptops and smartphones, as well as in light duty transport devices like drones and kickboards.
Currently, liquid fuel cells that generate electricity through the oxidation of organic compounds such as methanol, ethanol, formic acid, and formate have halted commercialization due to output limitations.
Recently, performance improvement research on alkaline liquid fuel cells using palladium catalysts has been conducted; however, despite no carbon monoxide intermediates being generated during formate oxidation, research is still needed to resolve factors hindering performance improvement.
In particular, controlling the desorption reaction of hydrogen adsorbates, which is the rate-determining step in the reaction mechanism, is important. The research team confirmed that by incorporating boron (B) into the palladium lattice and effectively improving the electronic structure between palladium and boron, the hydrogen adsorption strength was weakened, resulting in more than a 50% enhancement in the formate oxidation reaction.
Professor Jae-young Lee said, “We secured the world’s top performance in fuel cells by developing a catalyst utilizing the oxidation characteristics of formate, an eco-friendly fuel that can be mass-produced from carbon dioxide,” and added, “We expect that follow-up research will make a significant contribution to the advancement of next-generation energy conversion technologies.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.