KAIST Professor Kim Yoo-sik's Research Team Uncovers Mechanism of DNA Demethylation Agents
Effect Varies Depending on Specific Gene Expression
Step Forward in 'Personalized' Cancer Treatment... Potential to Save Patient Costs and Time
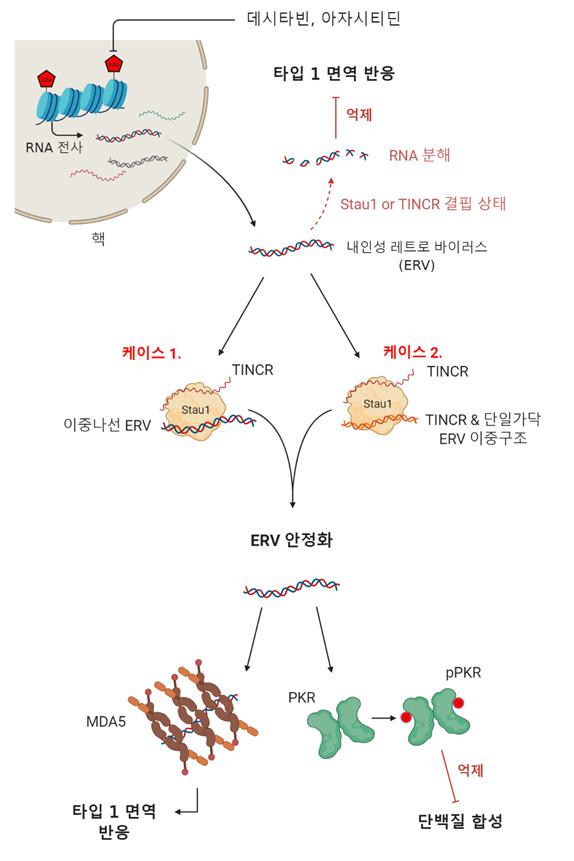 Schematic diagram of the mechanism of chemical chemotherapy using DNA demethylating agents. Differences in in vivo immune responses according to the expression of Staufen1 and TINCR. Image courtesy of KAIST.
Schematic diagram of the mechanism of chemical chemotherapy using DNA demethylating agents. Differences in in vivo immune responses according to the expression of Staufen1 and TINCR. Image courtesy of KAIST.
[Asia Economy Reporter Kim Bong-su] Domestic researchers have identified the mechanism of action of a specific anticancer drug used for acute myeloid leukemia (AML) and other conditions. This discovery enables distinguishing the drug's effectiveness on an individual basis according to genetic factors, allowing for personalized treatment of patients and saving economic costs and time.
The Korea Advanced Institute of Science and Technology (KAIST) announced on the 7th that Professor Kim Yoo-sik's research team from the Department of Biological Sciences and Bioengineering collaborated with Professor Hong Joon-sik's team from the Seoul National University Hospital Blood Cancer Center to identify key factors involved in anticancer chemotherapy.
The anticancer agent whose effectiveness was confirmed according to genetic factors is decitabine, a chemotherapy drug used for acute myeloid leukemia and myelodysplastic syndrome. DNA demethylating agents like decitabine participate in the DNA replication process and remove methyl groups (-CH₃) present on DNA to regulate gene expression. Notably, cancer cells have a higher amount of methylated DNA compared to normal cells, which suppresses the transcription process that generates RNA from DNA.
However, many patients treated with DNA demethylating agents do not respond to the drug, and this could not be determined prior to administration.
The research team analyzed various double-stranded RNA (dsRNA) binding proteins that interact with dsRNA regulated by DNA demethylating agents and for the first time identified that 'Staufen1,' a protein that directly binds to dsRNA and regulates its stability, plays a crucial role in cellular responses to decitabine. In cells where Staufen1 expression was suppressed, dsRNA was rapidly eliminated, resulting in no downstream immune response and no observed cancer cell death.
The team analyzed the expression patterns of the Staufen1 gene in bone marrow extracts from 46 patients with acute myeloid leukemia and myelodysplastic syndrome treated with DNA demethylating agents such as decitabine and azacitidine. They confirmed that patients who did not respond to the drug showed significantly reduced expression of Staufen1. Patients with low Staufen1 expression had lower overall survival and progression-free survival rates, indicating a poor prognosis.
Professor Kim Yoo-sik of KAIST's Department of Biological Sciences and Bioengineering stated, "This study not only elucidated the mechanism of action of the decitabine anticancer drug but also validated its effect in samples from patients who actually received decitabine." He added, "In the future, biomarker development based on the identified gene will allow prediction of the effectiveness of DNA demethylating agents such as decitabine and azacitidine, which will be useful in establishing effective personalized cancer treatment strategies."
The research results were published on the 30th of last month in the international journal Proceedings of the National Academy of Sciences (PNAS).
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















