Korea Institute of Materials Science Research Team Succeeds in Creating Non-Metallic Material Catalysts Instead of Expensive Precious Metals
[Asia Economy Reporter Kim Bong-su] A domestic research team has developed a technology that can produce hydrogen cheaply and environmentally friendly using seawater without any by-products. Although hydrogen is gaining attention as the next-generation renewable energy source, such as in hydrogen fuel cell vehicles, the current production methods and technology levels are costly and generate a lot of pollutants, making them no different from traditional energy sources like petroleum and nuclear power. It is now drawing attention whether these limitations can be overcome.
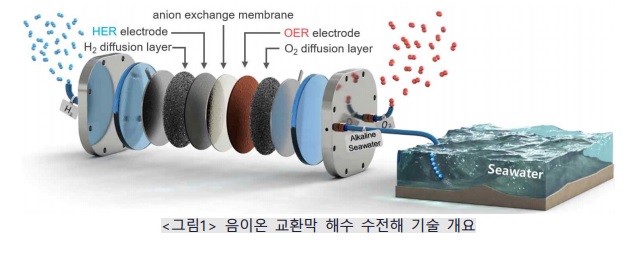
On the 15th, the Korea Institute of Materials Science (KIMS) announced that the research team led by Dr. Choi Seung-mok and Dr. Lee Ji-hoon from the Energy Electronic Materials Laboratory developed the "anion exchange membrane seawater electrolysis technology," which can drastically reduce hydrogen production costs by directly producing green hydrogen using seawater, the most abundant water resource on Earth, for the first time in Korea.
The research team succeeded in producing high-purity hydrogen by electrolyzing seawater, the most abundant water on Earth, by developing the core technologies of seawater electrolysis: "highly selective oxygen evolution reaction control technology" and "chlorine evolution reaction suppression technology." Among the electrolysis technologies suitable for green hydrogen production, "anion exchange membrane electrolysis" is a technology that safely produces high-purity hydrogen at low cost using inexpensive non-precious metal catalysts. However, it has been expensive and difficult to popularize because purified water (ultrapure water) must be used for hydrogen production.
The research team developed a catalyst-integrated electrode by directly forming a nickel (Ni)-doped, highly selective two-dimensional nanosheet-shaped iron oxyhydroxide (FeOOH) catalyst on the electrode surface. This reduced the overpotential of the oxygen evolution reaction and mass transfer resistance. By applying and optimizing this electrode in anion exchange membrane electrolysis, they suppressed the chlorine evolution reaction and secured high hydrogen generation efficiency.
Generally, when hydrogen is produced by directly electrolyzing seawater, hydrogen can be obtained at the reduction electrode, but at the opposite oxidation electrode, chloride ions contained in seawater are oxidized to produce chlorine in the "chlorine evolution reaction," and water is oxidized to produce oxygen in the "oxygen evolution reaction," which compete with each other. At this time, the electrode surface becomes locally acidic due to the chlorine evolution reaction, which requires the use of expensive precious metals (platinum (Pt), iridium (Ir), palladium (Pd), etc.) that are stable in acidic conditions as electrode catalyst materials, which is a disadvantage.
However, the research team controlled the pH of seawater to raise the potential at which the chlorine evolution reaction occurs from 1.36 V (vs. RHE) to 1.72 V (vs. RHE), suppressing the reaction. By applying the developed highly active catalyst to reduce overpotential, the oxygen evolution reaction predominates, allowing the use of non-precious metal catalyst materials.
Dr. Choi Seung-mok, the lead researcher, said, "Through the non-precious metal-based anion exchange membrane seawater electrolysis technology, we have opened the way to produce high-purity hydrogen cheaply using seawater." He added, "In particular, if green hydrogen is produced in connection with marine renewable energy such as offshore wind power, it is expected to create a new business model where hydrogen can be directly charged onto hydrogen ships at sea."
The research results were published as the cover paper in the June 3 issue of the Journal of Materials Chemistry A, a journal of the Royal Society of Chemistry (RSC) in the UK. The research team is currently conducting demonstration research applying these results to an anion exchange membrane seawater electrolysis system.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.