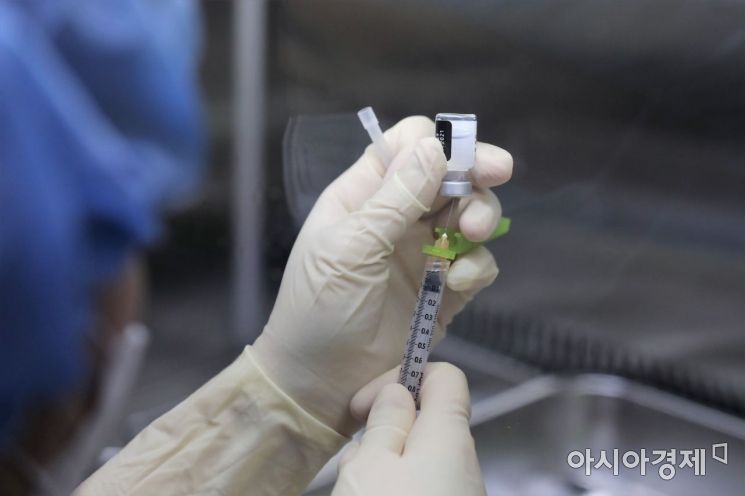
 On the morning of the 27th of last month, when Pfizer vaccine administration began for medical staff treating COVID-19 patients, medical personnel were preparing doses of the Pfizer vaccine in syringes at a sterile workstation (clean bench) inside the Central Vaccination Center of the National Medical Center in Jung-gu, Seoul. Photo by Joint Press Corps
On the morning of the 27th of last month, when Pfizer vaccine administration began for medical staff treating COVID-19 patients, medical personnel were preparing doses of the Pfizer vaccine in syringes at a sterile workstation (clean bench) inside the Central Vaccination Center of the National Medical Center in Jung-gu, Seoul. Photo by Joint Press Corps
[Asia Economy Reporter Lee Chun-hee] The final decision on the formal approval of Pfizer's COVID-19 vaccine 'Comirnaty' will be made on the 5th.
The Ministry of Food and Drug Safety (MFDS) announced on the 4th that it will hold a final review committee meeting for the Pfizer vaccine at 10 a.m. on the 5th in the MFDS conference room in Osong, Cheongju, Chungbuk. The results of the meeting are scheduled to be disclosed through a briefing at 2 p.m. on the same day.
The MFDS currently conducts a three-tier advisory review process before approving COVID-19 vaccines and treatments, involving a verification advisory group, the Central Pharmaceutical Affairs Deliberation Committee, and the final review committee. Previously, the verification advisory group and the Central Pharmaceutical Affairs Committee judged that Pfizer vaccine's preventive efficacy of 95% is sufficient for approval.
Along with this, they expressed the opinion that vaccination could be approved not only for those aged 18 and over but also for adolescents aged 16 to 17. The safety profile, including adverse events, was also evaluated to be at an acceptable level.
The MFDS will comprehensively review these advisory opinions, Pfizer vaccine's efficacy and effectiveness, dosage and administration, recommendations, and additional quality data to make the final approval decision at the final review committee meeting on the day.
Previously, the MFDS announced that it would significantly shorten the approval review period for COVID-19 vaccines and treatments to 40 days. Traditionally, it took more than six months to obtain product approval. Since Korea Pfizer applied for product approval on January 25, the approval decision should be finalized within this week according to the schedule.
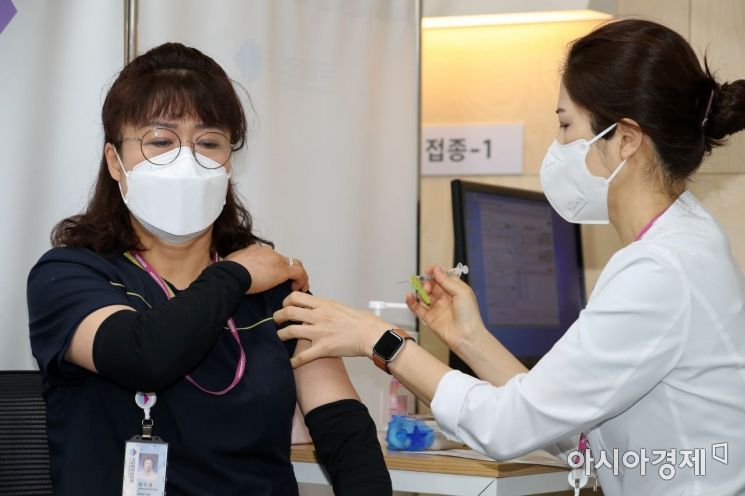 On the morning of the 27th of last month, a medical center official, the first recipient of the Pfizer vaccine, is receiving the vaccination at the Central Vaccination Center of the National Medical Center in Euljiro, Jung-gu, Seoul. Photo by Joint Press Corps
On the morning of the 27th of last month, a medical center official, the first recipient of the Pfizer vaccine, is receiving the vaccination at the Central Vaccination Center of the National Medical Center in Euljiro, Jung-gu, Seoul. Photo by Joint Press Corps
Currently, the Pfizer vaccine has been administered domestically to COVID-19 treatment medical staff since the 27th of last month. This supply was introduced through the international vaccine joint procurement project 'COVAX Facility' and is being administered under a 'special import' approval that allows the import of pharmaceuticals without formal domestic approval.
This product approval pertains to 13 million doses contracted directly between our government and Pfizer, and since it is a separate supply quantity, it requires separate approval apart from the special import procedure. The government plans to introduce 500,000 doses starting at the end of this month and 3 million doses in the second quarter.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
















