[Asia Economy Reporter Kim Bong-su] The Korea Advanced Institute of Science and Technology (KAIST) announced on the 3rd that it has developed a battery material capable of storing more than 20% more electricity than current materials.
The research team led by Professor Eun-ae Cho from the Department of Materials Science and Engineering at KAIST discovered a high-capacity lithium-excess cathode material that has an energy density over 20% higher than the currently used battery cathode materials while maintaining stability.
Currently, electric vehicle batteries use high-nickel (Ni) cathode materials. Battery cathode materials are oxides of cobalt (Co), nickel (Ni), and manganese (Mn), and the higher the nickel content, the greater the capacity (200mAh/g).
The problem is that high-nickel cathode materials show limitations in improving driving range. In response, the research team proposed lithium-excess cathode materials as an alternative to high-nickel cathode materials. Lithium-excess cathode materials are next-generation cathode materials containing an excess amount of lithium, with a stored lithium amount that allows a usable capacity of 250mAh/g, enabling storage of 20% more energy than conventional high-nickel cathode materials.
However, lithium-excess cathode materials undergo a reaction in which oxygen, which constitutes the oxide, irreversibly evolves as gas between the first charge and discharge. As a result, the structure of the oxide cathode material collapses, and battery performance rapidly deteriorates, preventing their use.
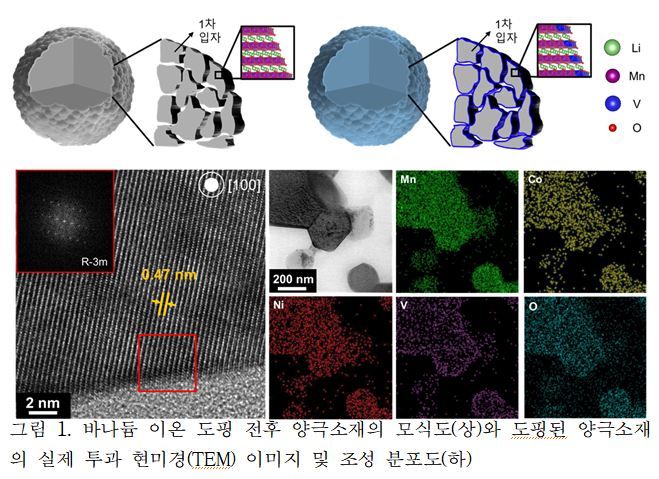
Professor Eun-ae Cho’s research team developed a technology to selectively dope vanadium (V) ions on the cathode surface where irreversible oxygen reactions mainly occur, successfully enhancing the stability of lithium-excess cathode materials. While lithium-excess cathode materials exhibit a low reversibility of 69% during the first charge and discharge, vanadium-doped lithium-excess cathode materials showed a high reversibility of 81% during the first charge and discharge and maintained stability of up to 92% even after 100 charge-discharge cycles.
Professor Eun-ae Cho explained, "The doped vanadium ions change the electronic structure of oxygen ions within the cathode material, enabling reversible oxidation-reduction reactions during charge and discharge," adding, "The overall process is relatively simple, making it suitable for mass production."
These research results were published online on January 29 in the prestigious international journal in the field of materials science, 'Advanced Science.'
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.