[Asia Economy Reporter Seo So-jung] Celltrion continued its record-breaking performance.
On the 22nd, Celltrion announced through its business performance disclosure that its consolidated sales last year reached 1.8491 trillion KRW, and operating profit was 712.1 billion KRW, marking increases of 63.9% and 88.4% respectively compared to the previous year.
In the fourth quarter of last year, sales were 498.7 billion KRW and operating profit was 164.7 billion KRW, up 30.3% and 44.3% year-on-year, respectively.
Celltrion explained that last year, the expansion of its biosimilar product lineup increased supply volume, and the production efficiency of the expanded facilities at its first plant improved, resulting in favorable performance.
Regarding its main product lineup, as of the third quarter of last year, market shares in Europe were recorded as Remsima (52.8%), Truxima (38%), and Herzuma (15.9%). In the United States, as of the fourth quarter of last year, market shares of Inflectra (11.8%) and Truxima (19.8%) showed growth trends.
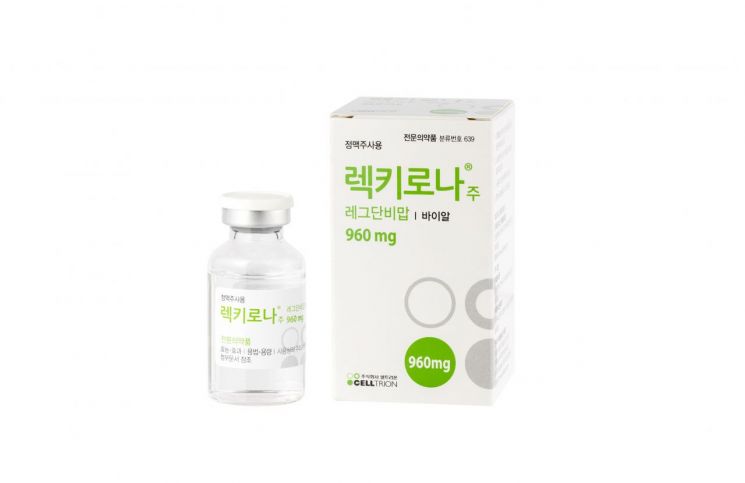
This year, Celltrion plans to focus on expanding the development of follow-up biosimilars, global approval expansion of the COVID-19 antibody treatment 'Rekkirona,' accelerating market penetration of Remsima SC, and increasing production capacity through the establishment of a third plant.
In February this year, Celltrion received sales approval from the European Commission (EC) for 'Yuflyma (CT-P17),' a high-concentration Humira biosimilar, thereby securing a competitive product lineup. Additionally, it is expanding the development of follow-up biosimilars including CT-P16 (Avastin biosimilar), CT-P39 (Xolair biosimilar), CT-P41 (Prolia biosimilar), CT-P42 (Eylea biosimilar), and CT-P43 (Stelara biosimilar), all currently undergoing global Phase 3 clinical trials, with plans to obtain approval for at least one product annually until 2030.
Celltrion obtained conditional approval for the COVID-19 antibody treatment Rekkirona from the Ministry of Food and Drug Safety in February this year, and plans to secure approvals through emergency use authorization and conditional approvals in the United States and Europe within the first half of the year. Celltrion has completed production of treatment doses for 100,000 domestic patients and plans to produce an additional 1.5 to 3 million doses annually depending on demand.
To respond to variant viruses, Celltrion is accelerating the establishment of a variant response platform and has also begun developing a cocktail therapy combining candidate antibody No. 32, which showed neutralizing ability against the UK and South African variants, with Rekkirona.
Furthermore, Celltrion plans to secure stable production capacity by actively advancing the construction of its third plant and Global Biotechnology Research Center on a site in Songdo New City, Yeonsu-gu, Incheon, which began in November last year. The third plant is scheduled for completion in May 2023, and the research center in July 2022. Commercial production at the third plant is expected to commence in June 2024, and upon completion, Celltrion will secure a total annual production capacity of 250,000 liters, adding to the existing 190,000 liters from plants 1 and 2.
A Celltrion official stated, "Despite the COVID-19 pandemic last year, our main product lines grew steadily, enabling us to continue our record-breaking performance. This year, with global supply efforts for the COVID-19 antibody treatment and the full-scale development and new supply of follow-up biosimilars such as Yuflyma (CT-P17), a Humira biosimilar, we will continue to grow as a global biotechnology company."
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















