[Asia Economy Honam Reporting Headquarters Reporter Lee Gwanwoo] The Gwangju Institute of Science and Technology (GIST) announced on the 2nd that a joint research team consisting of Professor Jaeseok Lee and Professor Joohyeong Lee from the Department of Materials Science and Engineering and Professor Chanho Park from the Graduate School of Energy Convergence confirmed that functional groups within polymer electrolyte membranes affect the leakage of phosphoric acid, and through this, proposed a method to improve the performance of high-temperature polymer electrolyte membrane fuel cells.
A functional group refers to a common atomic group bonding pattern in a group of organic compounds that share common chemical characteristics and are responsible for those characteristics.
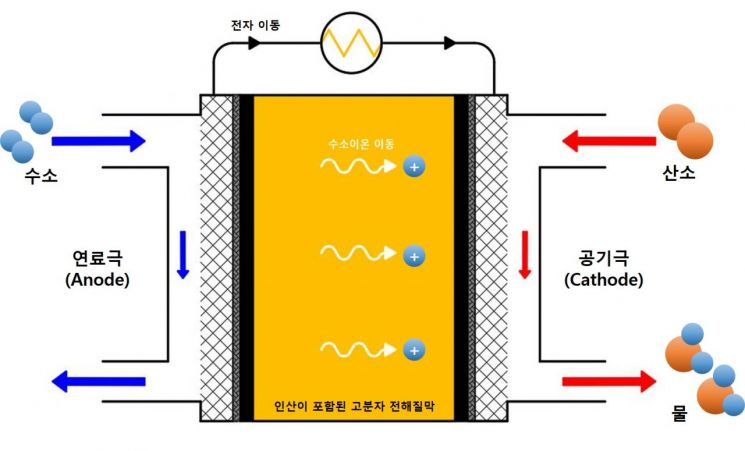
Phosphoric acid is a compound composed of phosphorus (P), four oxygen (O) atoms, and three hydrogen (H) atoms, exhibiting acidity and facilitating the movement of hydrogen ions in high-temperature polymer electrolyte membrane fuel cells.
The research team optimized the functional groups within the electrolyte membrane to minimize phosphoric acid leakage and elucidated the leakage phenomenon through computational science.
It is expected to be utilized in the development of high-efficiency next-generation building fuel cells in the future.
High-temperature polymer electrolyte membrane fuel cells operating at temperatures above 120°C have the advantage of utilizing waste heat for hot water and heating, making them a focus as next-generation building-type fuel cell systems.
These fuel cells move hydrogen ions through phosphoric acid within the electrolyte membrane, but during operation, phosphoric acid leaks from the electrolyte membrane to the electrodes, causing a decline in fuel cell performance.
To investigate the phosphoric acid leakage phenomenon, the research team manufactured and compared polymer electrolyte membranes incorporating three different azole compounds.
Azole compounds are relatively basic compared to phosphoric acid, allowing phosphoric acid to be retained within the electrolyte membrane through acid-base interactions.
They also studied the effects of nitrogen substituents and basicity of azole compounds substituted with hydrogen and methyl groups on phosphoric acid leakage in the electrolyte membrane.
When the electrolyte membrane containing phosphoric acid was exposed to moisture, the methylimidazole electrolyte membrane, which showed the least phosphoric acid leakage (6.9% leakage), was applied to the fuel cell, resulting in the best performance (0.197 W/cm2).
Additionally, by calculating the bonding forces among phosphoric acid, azole compounds, and water molecules within the electrolyte membrane through Fourier transform analysis, they confirmed consistency with experimental results, theoretically elucidating the phosphoric acid leakage phenomenon in the electrolyte membrane.
Hydrogen ion conduction through phosphoric acid occurs via the Grotthuss mechanism, where hydrogen ions hop between phosphoric acid molecules. When methyl groups are present on azole compounds in the polymer electrolyte membrane, the amount of acidic hydrogen decreases, reducing the efficiency of the Grotthuss pathway and hydrogen ion conductivity; however, moisture absorption is lower, resulting in less phosphoric acid leakage and thus improved hydrogen fuel cell performance.
The Grotthuss pathway is a process where excess hydrogen ions or hydrogen ion defects in neighboring molecules diffuse through a hydrogen bond network by repeatedly forming and breaking covalent bonds.
Professor Jaeseok Lee stated, “This research derived a direct performance improvement method for high-temperature polymer electrolyte membrane fuel cells, which are gaining attention as next-generation building fuel cells, through the convergence of materials, unit cells, and computational science.” He added, “As fuel cell adoption expands in homes, it is expected to contribute to promoting an eco-friendly hydrogen economy by utilizing waste heat for heating and hot water alongside distributed power generation.”
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















