SK Bio to Handle Distribution... Final Review of Vaccination Plan Today
[Asia Economy Reporter Seo So-jeong] As the novel coronavirus disease (COVID-19) vaccine is expected to be introduced domestically before the Lunar New Year holiday, the government has begun full-scale preparations to start vaccinations. On the afternoon of the 22nd, the Korea Disease Control and Prevention Agency (KDCA) will hold a Vaccination Expert Committee meeting to refine plans regarding priority vaccination groups for the COVID-19 vaccine and finalize the plan after expert review.
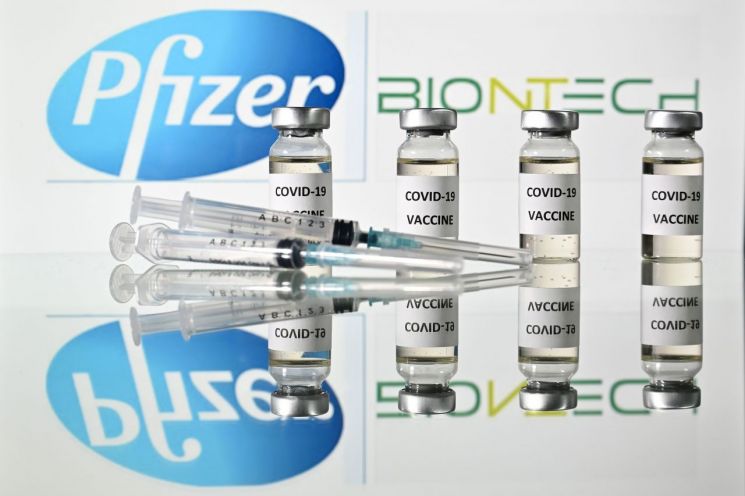
The initial quantity of the Pfizer vaccine, which is likely to be introduced before the Lunar New Year holiday, is 50,000 doses. Pfizer Korea plans to submit an application for COVID-19 vaccine approval to the Ministry of Food and Drug Safety within this month. Due to the difficulty of distributing and storing the Pfizer vaccine at an ultra-low temperature of around minus 70 degrees Celsius, the government will establish about 250 vaccination centers nationwide to begin vaccinations separately.
Preparations for distribution are also underway. The KDCA announced a public notice to the Public Procurement Service for the ‘Establishment and Operation of Domestic Distribution Management System for COVID-19 Vaccines’ and selected SK Bioscience as the distributor. The allocated budget is 51 billion KRW, and the project amount is 50.8 billion KRW.
An SK Bioscience official stated, "We will be responsible for vaccine distribution, storage, and cold chain establishment." According to the vaccine type, the government plans to administer the Pfizer and Moderna vaccines, which require frozen storage, at separate vaccination centers, while AstraZeneca and Janssen vaccines, which can be stored refrigerated at room temperature, will be administered at entrusted medical institutions.
Detailed Check of Pre-vaccination Screening Form... Must Record Vaccination Status and Date
Ahead of full-scale vaccinations next month, the COVID-19 Vaccine Subcommittee reviewed the draft of the ‘pre-vaccination screening form.’ Like the influenza (flu) vaccine, a pre-vaccination screening form must be completed before receiving the COVID-19 vaccine.
The screening form has added items such as ‘vaccination status and date of COVID-19 vaccine’ and ‘presence of allergic reactions’ to the existing sections. This is because most COVID-19 vaccines require two doses and the vaccination schedule must be followed to form immunity. This also helps prevent ‘heterologous vaccination,’ where a person receives different vaccines.
However, the medical community has advocated for the digitization of the screening form. Since the entire population will be vaccinated, the existing paper system could cause inconvenience for medical staff and patients.
Ji Young-mi, a vaccination expert and advisor at the Seoul National University College of Medicine Global Infectious Disease Center, said, "The Vaccination Expert Committee is scheduled to hold a final review of the vaccination plan this afternoon."
Meanwhile, as of midnight on the same day, the number of confirmed COVID-19 cases in Korea was 346, dropping back to the 300s after three days.
© The Asia Business Daily(www.asiae.co.kr). All rights reserved.
![Clutching a Stolen Dior Bag, Saying "I Hate Being Poor but Real"... The Grotesque Con of a "Human Knockoff" [Slate]](https://cwcontent.asiae.co.kr/asiaresize/183/2026021902243444107_1771435474.jpg)















